A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
External Cephalic Version: Is it an Effective and Safe Procedure?
In This Article
Erratum Notice
Summary
This article shows how to perform the external cephalic version (ECV) by two experienced obstetricians in the obstetric operating room in the presence of an anesthesiologist and a midwife. The ECV is carried out with analgesia and tocolysis. Two attempts are made under ultrasonography control.
Abstract
External cephalic version (ECV) is an effective procedure for reducing the number of cesarean sections. To date, there is no video publication showing the methodology of this procedure. The main objective is to show how to perform ECV with a specific protocol with tocolysis before the procedure and analgesia. Moreover, we describe and analyze the factors associated with successful ECV, and also compare to deliveries in the general pregnant population.
A retrospective and descriptive analysis of ECV carried out at the Hospital Clinico Universitario Virgen de la Arrixaca in Murcia (Spain) between 1/1/2014 and 12/31/2018 was assessed. The latest data available of labor deliveries in the local center, which is the biggest maternity department in Spain, were from 2018.
320 patients were recruited and 3 pregnant women were lost during the study. ECV was carried out at 37±3 weeks gestation. ECV was successful in 82.5% (N=264). 19 complications were reported (5.9%): 8 vaginal bleeding (2.5%), 9 fetal bradycardia (2.8%), 1 preterm rupture of membranes (0.3%) and 1 cord prolapse (0.3%). A previous vaginal delivery increases the success rate of ECV ORadjusted=3.03 (1.62-5.68). Maternal Body Mass Index (BMI) affects the success of ECV ORadjusted=0.94 (0.89-0.99). Patients with BMI>40 kg/m2 have an ORadjusted=0.09 (0.009-0.89) compared with those with BMI <25 kg/m2. If ECV was successful, the cesarean delivery index is 22.2% (17.5-27.6%), the eutocic delivery index is 52.1% (46.1-58.1%) and the instrumented vaginal delivery index is 25.7% (20.7-31.2%). There are no differences in cesarean and eutocic delivery indexes after successful ECV. However, a successful ECV is associated with a 6.29% increase in the instrumented delivery rate (OR=1.63).
ECV is an effective procedure to reduce the number of cesarean sections for breech presentations. Maternal BMI and previous vaginal delivery are associated with ECV success. Successful ECV does not modify the usual delivery pattern.
Introduction
External cephalic version (ECV) is a procedure for modifying the fetal position and achieving a cephalic presentation. The objective of the ECV is to offer an opportunity for cephalic delivery to occur, which is widely known to be safer than breech or cesarean section. ECV is usually performed before the active labor period begins. Factors associated with a higher ECV success rate include1,4: multiparity, a transverse presentation, black race, posterior placenta, amniotic fluid index higher than 10 cm.
However, ECV is not an innocuous procedure and may present7,11 intraversion complications such as premature rupture of membranes, vaginal bleeding, transitory changes of fetal heart rate, cord prolapse, abruptio placentae, even stillbirth.
In this article, we analyzed ECVs performed under analgesia and tocolysis. To date, no video report has been published showing how to perform this procedure with analgesia and tocolysis. The main objective of this study is to show how ECV is performed. We also describe some key actions that could improve the procedural success. As a secondary objective, we analyzed the results of ECV obtained following the specific protocol with tocolysis and analgesia and compared the results with the literature. We also analyzed factors associated with ECV success rate, type of delivery and a comparison of the type of delivery in ECV pregnant women with non-ECV pregnant women were also included.
Access restricted. Please log in or start a trial to view this content.
Protocol
This study was approved by the Clinic Research Committee of the "Virgen de la Arrixaca" at the University Clinical Hospital. Written informed consent was obtained from all participants.
1. Offer external cephalic version at consult (36 weeks)
- Identify the fetal position with an ultrasound.
- Apply ultrasound gel on the patient’s abdomen.
- Place the abdominal probe on the hypogastric region.
- Identify the fetal position.
- Offer external cephalic version.
- Have the patient sign informed consent.
2. Admit in the obstetric emergency room (≥37 weeks)
- Identify the fetal position with an ultrasound.
- Apply ultrasound gel on the patient’s abdomen.
- Place the abdominal probe on the hypogastric region.
- Identify the fetal position.
- Prepare the patient and check requirements (blood test and informed consent).
3. Admit in the obstetric delivery room
- Perform cardiotocography assessment for fetal well-being.
- Add 10 mL of ritodrine in 500 mL of glucose solution.
- 30 minutes before the procedure, administer 6 mg of ritodrine at 60 mL/h.
- Invite the patient to empty her bladder.
- Stop ritodrine perfusion before moving to the obstetric operating room.
4. External cephalic version procedure in obstetric operating room
- Move to the obstetric operating room.
- Monitor maternal vital signs (heartrate, EKG, temperature, noninvasive blood pressure, oxygen saturation).
- Identify the fetal position with an ultrasound.
- Apply ultrasound gel on the patient’s abdomen.
- Place the abdominal probe on the hypogastric region.
- Identify the fetal position.
- Position the patient in Trendelenburg (15°).
- Perform analgesia.
- Sedate the patient with 1-1.5 mg/kg propofol IV.
- Alternatively: Provide spinal anesthesia with 10 mL of 0.1% bupivacaine.
- External cephalic version procedure (2 attempts)
- Apply an abundant quantity of ultrasound gel on the patient’s abdomen.
- Place hands in the hypogastric region to identify the fetal buttocks (Obstetrician A).
- Elevate fetal buttocks (Obstetrician A).
- Place hands in the patient’s abdomen to locate the fetal head (Obstetrician B).
- Guide the fetal buttocks to the fundus (Obstetrician A).
- Consecutively direct the fetal head to the pelvis (Obstetrician B).
- Identify the fetal well-being with ultrasound.
- Place the abdominal probe on the abdomen.
- Identify the fetal heart.
- Observe the fetal heart rate for at least a minute.
- Check for vaginal bleeding.
- Identify the fetal position with ultrasound.
- Place the abdominal probe on the hypogastric region.
- Identify the fetal position.
5. Move to obstetric delivery room
- Perform cardiotocography assessment for fetal well-being for at least 4 hours.
- Discharge the patient.
6. Admit in obstetric emergency room (24 h post-procedure)
- Perform cardiotocography assessment for fetal well-being for 30 minutes.
Access restricted. Please log in or start a trial to view this content.
Results
Three hundred and twenty patients were recruited between January 1, 2014 and December 31, 2018. Three patients were lost during the follow-up after the ECV because delivery was not carried out in our hospital.
Statistics were derived from the raw data. To study the differences between groups, unpaired Student's t-tests were used for quantitative variables and chi-square tests for dichotomous variables. All tests were two-tailed at a 0.05 significance level.
The ...
Access restricted. Please log in or start a trial to view this content.
Discussion
This article shows the procedure to carry out the ECV. The ECV procedure success rate in this study is 82.5% (78.1-86.4%), which is higher than the success rate found in international literature 49.0% (47-50.9%)1,2,3,4 or Spanish literature 53.49% (42.9-64.0%)5,6,7. This difference may be due to the us...
Access restricted. Please log in or start a trial to view this content.
Disclosures
The authors have nothing to disclose.
Acknowledgements
We thank all the midwives and anesthesiologists who have collaborated on this project. David Simó has specially collaborated with this project recording the procedure.
Access restricted. Please log in or start a trial to view this content.
Materials
| Name | Company | Catalog Number | Comments |
| Avalon FM20 Fetal monitor | Koninklijke Philips N.V | https://www.usa.philips.com/healthcare/product/HC862198/avalon-fm20-fetal-monitor | |
| Convex Array Probe 4C-RS | General Electric Health Care Company | ||
| Aquasonic® 100 Ultrasound Transmission Gel | Parker Laboratories, INC | https://www.parkerlabs.com/aquasonic-100.asp | |
| Propofol Lipuro (10 mg/mL Inject 20 mL) | B. Braun Medical, SA | ||
| Ritodrine Pre-par (10 mg/mL) | Laboratorio Reig Jofré, S.A | ||
| Viridia series 50 XM Fetal Monitor | Koninklijke Philips N.V | https://www.usa.philips.com/healthcare/product/HC865071/avalon-fm50-fetal-monitor | |
| Voluson P6 | General Electric Health Care Company | https://www.ge-sonostore.com/en/voluson/p6 |
References
- Melo, P., Georgiou, E. X., Hedditch, A., Ellaway, P., Impey, L. External cephalic version at term: a cohort study of 18 years' experience. British Journal of Obstetrics and Gynecology. 126, 493-499 (2019).
- Hofmeyr, G. J., Kulier, R., West, H. M. External cephalic version for breech presentation at term. Cochrane Database of Systematic Reviews. 4, 83(2015).
- Isakov, O., et al. Prediction of Success in External Cephalic Version for Breech Presentation at Term. Obstetrics and Gynecology. 133 (5), 857-866 (2019).
- Ebner, F., et al. Predictors for a successful external cephalic version: a single centre experience. Archives of Gynecology and Obstetrics. 293 (4), 749-755 (2016).
- Castañer-Mármol, A. Results of external cephalic version in our area without rutinary tocolysis. Review and implementation strategy. , Public Health, Science History and gynecology Department.(Alicante, Miguel Hernández University). Dissertation (2015).
- Burgos, J., et al. Probability of cesarean delivery after successful external cephalic version. International Journal of Gynecology & Obstetrics. 131 (2), 192-195 (2015).
- Muñoz, M., et al. External cephalic version at term: accumulated experience. Progresos de Obstetricia y Ginecología. 48 (12), 574-580 (2005).
- Velzel, J., et al. Atosiban versus fenoterol as a uterine relaxant for external cephalic version: randomised controlled trial. British Medical Journal. 356 (26), 6773(2017).
- Thissen, D., Swinkels, P., Dullemond, R. C., van der Steeg, J. W. Introduction of a dedicated team increases the success rate of external cephalic version: A prospective cohort study. European Journal of Obstetrics & Gynecology and Reproductive Biology. 236 (3), 193-197 (2019).
- Hindawi, I. Value and pregnancy outcome of external cephalic versión. Eastern Mediterranean Health Journal. 11 (4), 633-639 (2005).
- Levin, G., Rottenstreich, A., Weill, Y., Pollack, R. N. The role of bladder volume in the success of external cephalic version. European Journal of Obstetrics & Gynecology and Reproductive Biology. 230, 178-181 (2018).
- Vani, S., Lau, S. Y., Lim, B. K., Omar, S. Z., Tan, P. C. Intravenous salbutamol for external cephalic version. International Journal of Gynecology & Obstetrics. 104, 28-31 (2009).
- Sullivan, J. T., et al. A randomized controlled trial of the effect of combined spinal-epidural analgesia on the success of external cephalic version for breech presentation. International Journal of Obstetric Anesthesia. 18, 328-334 (2009).
- Weiniger, C. F., et al. Randomized controlled trial of external cephalic version in term multiparae with or without spinal analgesia. British Journal of Anaesthesia. 104 (5), 613-618 (2010).
- Grootscholten, K., Kok, M., Oei, S. G., Mol, B. W., van der Post, J. A. External cephalic version-related risks: a meta-analysis. Obstetrics and Gynecology. 112 (5), 1143-1151 (2008).
- Sociedad Española de Ginecología y Obstetricia. External cephalic version (updated March 2014). Progresos de Obstetricia y Ginecología. 58 (7), 337-340 (2015).
- Chaudhary, S., Contag, S., Yao, R. The impact of maternal body mass index on external cephalic version success. Journal of Maternal-Fetal and Neonatal Medicine. 32, 21-59 (2019).
- de Hundt, M., Velzel, J., de Groot, C. J., Mol, B. W., Kok, M. Mode of delivery after successful external cephalic version: a systematic review and meta-analysis. Obstetrics and Gynecology. 123 (6), 1327-1334 (2014).
- Krueger, S., et al. Labour Outcomes After Successful External Cephalic Version Compared With Spontaneous Cephalic Version. Journal of Obstetrics and Gynecology Canada. 40, 61-67 (2018).
- Kean, L. H., Suwanrath, C., Gargari, S. S., Sahota, D. S., James, D. K. A comparison of fetal behaviour in breech and cephalic presentations at term. British Journal of Obstetrics and Gynecology. 106, 1209-1213 (1999).
Access restricted. Please log in or start a trial to view this content.
Erratum
Formal Correction: Erratum: External Cephalic Version: Is it an Effective and Safe Procedure?
Posted by JoVE Editors on 3/17/2023. Citeable Link.
An erratum was issued for: External Cephalic Version: Is it an Effective and Safe Procedure?. Figure 1 was updated from:
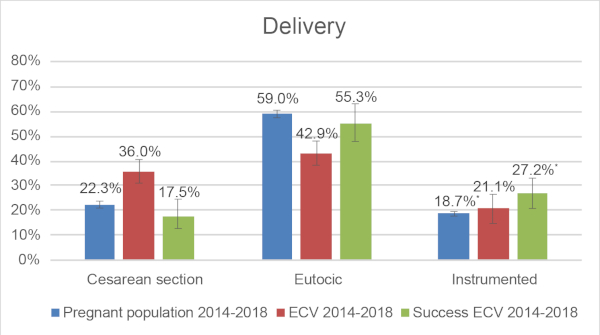
Figure 1: Comparison of type of delivery: General pregnant population in 2018, ECV cohort between 2014-2018, Successful ECV cohort between 2014-2018. * Chi-squared test: p<0.05. Please click here to view a larger version of this figure.
to:
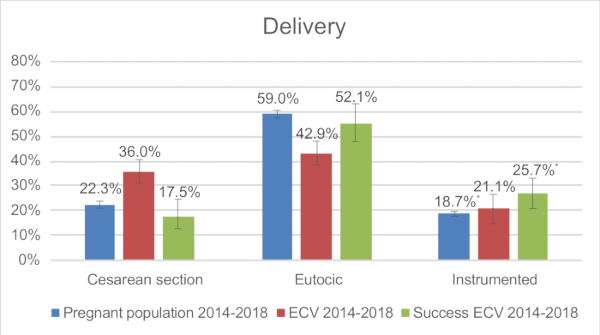
Figure 1: Comparison of type of delivery: General pregnant population in 2018, ECV cohort between 2014-2018, Successful ECV cohort between 2014-2018. * Chi-squared test: p<0.05. Please click here to view a larger version of this figure.
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved