A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Microscopy Techniques for Interpreting Fungal Colonization in Mycoheterotrophic Plants Tissues and Symbiotic Germination of Seeds
In This Article
Summary
This protocol aims to provide detailed procedures for collecting, fixing, and maintaining mycoheterotrophic plant samples, applying different microscopy techniques such as scanning and transmission electron microscopy, light, confocal, and fluorescence microscopy to study fungal colonization in plants tissues and seeds germinated with mycorrhizal fungi.
Abstract
Structural botany is an indispensable perspective to fully understand the ecology, physiology, development, and evolution of plants. When researching mycoheterotrophic plants (i.e., plants that obtain carbon from fungi), remarkable aspects of their structural adaptations, the patterns of tissue colonization by fungi, and the morphoanatomy of subterranean organs can enlighten their developmental strategies and their relationships with hyphae, the source of nutrients. Another important role of symbiotic fungi is related to the germination of orchid seeds; all Orchidaceae species are mycoheterotrophic during germination and seedling stage (initial mycoheterotrophy), even the ones that photosynthesize in adult stages. Due to the lack of nutritional reserves in orchid seeds, fungal symbionts are essential to provide substrates and enable germination. Analyzing germination stages by structural perspectives can also answer important questions regarding the fungi interaction with the seeds. Different imaging techniques can be applied to unveil fungi endophytes in plant tissues, as are proposed in this article. Freehand and thin sections of plant organs can be stained and then observed using light microscopy. A fluorochrome conjugated to wheat germ agglutinin can be applied to the fungi and co-incubated with Calcofluor White to highlight plant cell walls in confocal microscopy. In addition, the methodologies of scanning and transmission electron microscopy are detailed for mycoheterotrophic orchids, and the possibilities of applying such protocols in related plants is explored. Symbiotic germination of orchid seeds (i.e., in the presence of mycorrhizal fungi) is described in the protocol in detail, along with possibilities of preparing the structures obtained from different stages of germination for analyses with light, confocal, and electron microscopy.
Introduction
Structural research in botany, covering plant morphology and anatomy, is basic in understanding the whole organism1,2, and provides indispensable perspectives to integrate and contribute to knowledge regarding the ecology, physiology, development, and evolution of plants3. Methods in plant morphology and anatomy currently comprise protocols, equipment, and knowledge developed recently as well as more than a century ago2. The continuous execution and adaptation of classical methods (e.g., light microscopy) along with more recent techniques (e.g., confocal microscopy, X-ray microtomography) have the same essential basis: theoretical knowledge enabling the development of a methodology.
The main tool in plant anatomy and morphology is the image. Despite the misconception that such analyses are simple observations, giving space to subjective interpretations2, analyzing and understanding images in this area requires knowledge of the methods applied (the equipment, type of analysis, methodological procedures), cell components, histochemistry, and the plant body (tissue organization and function, ontogeny, morphological adaptations). Interpreting the images obtained via a variety of methods can lead to correlating form and function, deciphering the chemical composition of a structure, corroborating in describing taxa, understanding infections by phytopathogens, and other such assessments.
When researching mycoheterotrophic (MH) plants (i.e., non-photosynthetic plants that obtain carbon from mycorrhizal fungi4,5), remarkable aspects of their structural adaptations, the patterns of tissue colonization by fungi, and the morphoanatomy of subterranean organs can enlighten their development strategies and relationships with hyphae, which are the source of nutrients. The subterranean organs of MH plants usually show important adaptations related to their association with soil fungi, hence it is essential to perform these anatomical and morphological investigations6. MH species' aerial organs should not be ignored, as endophytes can be also present in these tissues, even if they are not mycorrhizal fungi (personal observations, not published yet).
Besides the well-established essentiality of mycorrhizal fungi association with MH species during their entire life cycle7, every orchid species, even the autotrophic ones, have an initial obligate mycoheterotrophic stage in natural environments. It occurs because the orchids' embryo is undifferentiated and lacks an endosperm or cotyledons, thus being incapable of developing and establishing itself in natural environments without the nutritional support of fungal partners4,8. Considering that, symbiotic germination protocols can be applied not only to MH species but also to photosynthesizing orchids, aiming to investigate orchid-fungus specificity in germination and protocorm development, a vastly applied methodology in initiatives for the conservation of threatened species9,10,11.
In this methods assembly, we describe important steps involved in collecting, fixing, and storing MH plant samples for anatomical studies (section 1), surface analysis and sample selection (section 2), sectioning methods (freehand: section 3, microtomy: section 4, cryomicrotomy: section 5), staining and mounting (section 6), fluorescence and confocal microscopy of fungal endophytes (section 7), scanning electron microscopy (section 8), and transmission electron microscopy (section 9). Additionally, we describe a symbiotic germination method for orchid seeds (MH and autotrophic, section 10), as the imaging methods previously mentioned can be successfully applied to analyze fungal colonization of seeds, protocorms, and seedlings in the germination process.
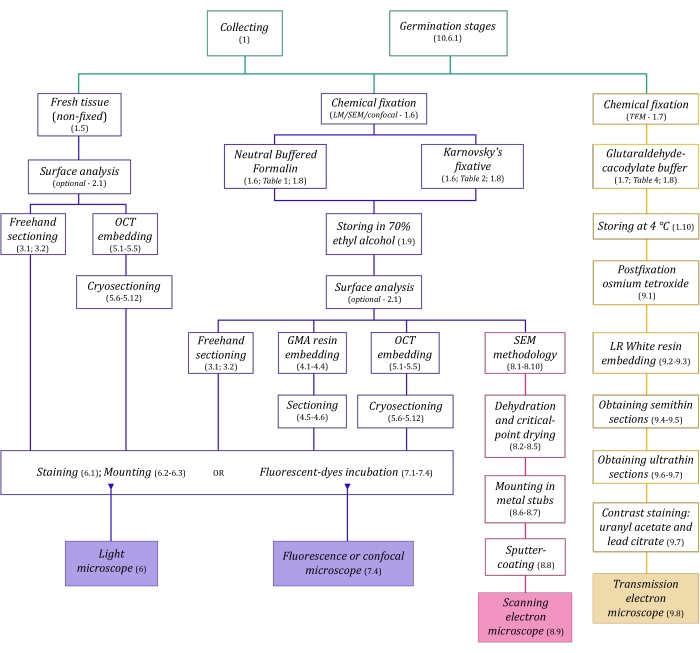
Figure 1: Schematic summarization of imaging methods. The schematics provide indications of protocol steps in which they are detailed. Abbreviations: GMA = glycol methacrylate, OCT = optimal cutting temperature compound, SEM = scanning electron microscopy. Please click here to view a larger version of this figure.
The microscopy techniques described here in detail (Figure 1) are preceded by the following essential steps: collecting, fixing, dehydrating, embedding, and sectioning samples. As the steps are variable (Figure 1) depending on the chosen technique(s), it is important to think ahead, considering the fixatives to be prepared and transported to the collection site, how the samples must be prepared before fixing, the dehydration processes to be used (section 1), and different embedding possibilities and sectioning methods (sections 4, 5, and 9). Figure 1 summarizes sequentially all the steps required for each microscopy technique thoroughly described below.
Access restricted. Please log in or start a trial to view this content.
Protocol
1. Collecting, fixing, and maintaining samples
NOTE: Fully MH plants can usually be found in the dark forest understory12,13, mainly in humid and litter-abundant areas, whereas partially MH plants can be found in more open forests12,13. MH plants usually have well-developed subterranean organs in a variety of shapes and sizes.
- When collecting MH species, explore the soil around the plant base, taking care not to damage underground organs, and avoid pulling the plants from the ground to prevent disconnecting the aerial organs from the subterranean ones.
- Carefully dig around aerial structures using a gardening trowel while exploring the subterranean organs like roots, stems, rhizomes, and storage organs, without damaging these structures.
- Remove soil particles to preserve fragile structures, and delicately wash these organs with tap water to rinse off the remaining soil particles before fixing the samples.
- MH plants associated with leaf litter demand extra attention; carefully collect organs connected to the decomposing material through their hyphae, avoid pulling these organs from the connected structures, and collect them carefully as these parts are highly delicate. Preserve structures with such connections and collect the litter as well for analysis.
- If opting to analyze fresh material using imaging techniques, maintain the samples in closed plastic bags with adequate moisture, enough water evaporating and moisturizing the plant, preventing excessive water being in contact with the samples. Transport them to the laboratory immediately and analyze the samples on the same day they were collected, while paying attention if the samples are still preserved when analyzing them.
- Carry fixatives to the collection site in well-sealed containers. Fix samples quickly after collection for light microscopy (LM) and scanning electron microscopy (SEM) in any of the following fixatives: 10% neutral buffered formalin (NBF14, Table 1) or Karnovsky's solution (modified15, Table 2). Karnovsky's solution can be prepared with 0.2 M phosphate buffer15, the recipe for which is described in Table 3.
- For analysis by transmission electron microscopy (TEM), section the samples with a thickness of 4-3 mm inside a drop of glutaraldehyde-sodium cacodylate buffer (modified16, Table 4) into smaller sections of 1-2 mm thickness. Discard the edges cut outside the drop. Immediately transfer the sections to a collection tube with a volume of fixative more than 10 times greater than the volume of the samples, as it is an additive fixative (i.e., its molecules are chemically added to the proteins fixed16).
CAUTION: The three described fixatives are highly toxic. Avoid inhalation, especially during their use in the field. Prepare all the fixatives in a fume hood using gloves. Do not mix cacodylate and acids, as arsenic gas can be formed16. - If the fixed samples float in the fixative, this indicates the presence of gas in plant tissues. Air and other gases prevent the fixative from penetrating the whole sample2. Remove gas from tissues by resampling them in smaller parts and using a vacuum pump (-300 to -400 inHg pressure) until all the samples sink to the bottom of the solution17. Be careful as excessive pressure exerted by the pump can damage samples.
- After at least 48 h in Karnovsky's solution or 10% NBF, wash the samples in 0.2 M PB (Table 3) and dehydrate them with a series of 10%, 30%, 50%, and finally 70% ethanol. For delicate samples, dehydrate for 30 min in each concentration; for larger samples, dehydrate for 1 h or longer.
NOTE: A 70% ethanol solution is the ideal medium for storing samples. Samples in 70% ethanol can be stored at room temperature for years. Do not store plant material for long periods in the fixatives, as removing fixing agents is an essential step after fixation2. - Store samples in glutaraldehyde-cacodylate at 4 °C before proceeding to postfixation (step 9.1).
| 10% neutral buffered formalin (NBF)14 | |
| step 1 | add 10 mL of 37-40% formaldehyde solution in 80 mL of distilled water |
| step 2 | add 0.4 g of sodium phosphate monobasic monohydrate (NaH2PO4·H2O) to the solution |
| step 3 | add 0.65 g of sodium phosphate dibasic, anhydrous (Na2HPO4) |
| step 4 | make up the volume to 100 mL |
Table 1: 10% neutral buffered formalin recipe14.
| Karnovsky's solution (modified15) | |
| step 1 | in 20 mL of distilled water at 60-70 °C |
| step 2 | add 0.8 g of paraformaldehyde (to obtain 4% w/v), stirring |
| step 3 | add 1-4 drops of 40% NaOH and stir until the solution becomes clear |
| step 4 | cool it and add 30 mL of 0.2 M phosphate buffer pH 7.2 (Table 3) |
| step 5 | dilute 25% glutaraldehyde in 0.1 M PB (pH 7.2) to obtain 1% glutaraldehyde (final volume: ~60 mL) |
| step 6 | add 1% glutaraldehyde (step 5) to the solution obtained in step 4 until making up to 100 mL of fixative |
Table 2: Karnovsky's solution recipe (modified15).
| 0.2 M phosphate buffer (PB) pH 7.2 | |
| step 1 | add 14.196 g of sodium phosphate dibasic, anhydrous (Na2HPO4) to 400 mL of distilled water |
| step 2 | add 13.8 g of sodium phosphate monobasic monohydrate (NaH2PO4·H2O) |
| step 3 | stir until the solution is clear |
| step 4 | adjust the final volume to 500 mL with distilled water |
| step 5 | adjust pH to 7.2 |
| step 6 | for a 0.1 M PB, dilute 1:1 |
Table 3: 0.2 M phosphate buffer recipe.
| 3% glutaraldehyde 0.2 M sodium cacodylate buffer (modified16) | |
| step 1 | 0.2 M cacodylate buffer: add 4.28 g of sodium cacodylate trihydrate in 100 mL of distilled water |
| step 2 | adjust pH to 7.2 |
| step 3 | add 12 mL of 25% glutaraldehyde in 25 mL of the solution in step 2 (0.2 M cacodylate buffer pH 7.2) |
| step 4 | make up the volume to 100 mL with distilled water |
Table 4: 3% glutaraldehyde 0.2 M sodium cacodylate buffer recipe (modified16).
2. Surface analysis of organs in fixed and non-fixed material
- To analyze superficial hyphae in organs, especially subterranean ones, and those in contact with leaf litter, observe fixed or fresh material in a dissecting microscope (stereomicroscope) at a magnification of 7.5x or above, depending on the samples analyzed.
- Visualize the samples immersed in the fixative, 70% ethanol (if stored in it), or tap water in the case of fresh material. Prevent direct light from the dissecting microscope as it can dry and damage samples.
- Search for areas of interest in the samples, guided by the superficial hyphae and rhizomorphs. Select samples which contain areas with superficial rhizomorphs as these can be sectioned to visualize pelotons and hyphae coils within cortical cells in roots and stems.
- After selection, follow steps 1.6 and 1.9 if the samples are not yet fixed. If desired, photograph fresh samples using a light microscope without fixing, as described in section 3.
- Use the camera coupled to the stereomicroscope to collect images from organ surfaces, rhizomorphs, and other structures observed. In such cases, arrange for an adequate background color to contrast well with the material and, if possible, choose a background material with a less rough surface (e.g., paper).
3. Freehand sections of plant organs
NOTE: Freehand sections of plant organs can be challenging, especially for small and thin structures. However, these sections of tissues with fungal endophytes can, in some cases, better evince hyphae and other features in comparison with thin sections.
- Section fresh or fixed samples with a sharp blade, cutting them as thin as possible and placing them immediately in a small Petri dish with water (if fresh) or 70% ethanol (if fixed). Use a small paintbrush to manipulate the sections without damaging them.
- To facilitate sectioning more challenging materials (i.e., small, thin, flexible organs), surround the sample in a structure, for instance, polystyrene or Cecropia petiole. Carve the support to accommodate the sample and make a thin section of the sample and the support altogether.
- Stain and mount the samples as described in section 6.
4. Embedding plant samples in resin and sectioning
- Further dehydrate the samples stored in 70% ethanol in 80%, 96%, and 2x in 100% ethanol, for 30 min to 2 h depending on the samples' size and composition.
- Use a glycol methacrylate (GMA) resin kit according to the manufacturer's instructions. Check Gerrits and Horobin (1996)18 for further considerations. Follow infiltration and embedding steps accordingly.
CAUTION: GMA resin is toxic, it can cause allergic reaction and skin, eyes, and mucosa irritation. Use the reagents in a fume hood and use gloves. - Use polyethylene molding trays for embedding, selected by samples size (e.g., 13 mm x 19 mm x 5 mm for larger samples, 6 mm x 8 mm x 5 mm for smaller ones). Pay attention to the desired sample orientation inside the mold, and use a needle to help orientate.
- Leave for polymerization, preferably at room temperature, until completely solidified. The hardening process usually takes a few hours, although it is recommended to unmold the blocks on the following day. After polymerization, carefully detach the resin blocks from the molds and proceed to attach the blocks as soon as possible to avoid block curving.
- Sand the face of the resin block that will be attached, creating a flat surface. Glue the resin block to a wooden cuboid (2 cm x 2 cm x 3 cm recommended) with a liquid cyanoacrylate adhesive of medium viscosity (see Table of Materials). Ensure that the resin is completely attached to avoid compromising sectioning.
- Perform sectioning in a rotary microtome as described below.
CAUTION: The blades of microtome knives are very sharp and can cause accidents. Be sure to handle them following all safety measures. Before approaching the knife (e.g., to change the block, to moist the resin), lock the coarse handwheel and place the blade safety cover on. Store disposable blades in appropriate cases. Be extremely careful when replacing blades.
NOTE: Different types of knives (e.g., disposable or fixed; glass or steel) can be used to section GMA resin18. The quality of the sections depends on how sharp the knife is. Make sure the knife is well attached and cannot move. Disposable knives may need to be regularly changed to achieve better sectioning.- Attach the wooden cuboid firmly to the block holder. Adjust the orientation of sectioning using the orientation screws and ensure an adequate angle of the knife using the knife tilt. Select the thickness of the sections; use a thicker setting for trimming and a thinner setting for selected sections, as GMA sections adhere more appropriately to the glass slide when thinner; suggested thickness for plant tissues is 5-8 µm.
- Before starting, cool the room, if necessary, as higher temperatures worsen the quality of sections. Prepare the following for sectioning: a beaker with distilled water, a hot plate, paintbrushes (at least two), fine point tweezers, a Pasteur pipette, glass slides, filter paper (or tissue paper), and a pencil (to identify the samples being sectioned).
- Adjust the hot plate to 50 °C and place the beaker on it. Pay attention to differences in heating depending on the area of the hot plate (usually, the middle area heats more than the edges; heat the water in the middle, preferably).
- Pick a glass slide, identify it with a pencil, and pipette the warm distilled water all over the slide surface. If necessary, use a solution (e.g., detergent and water, or 70% ethanol) to break the tension between the water and the glass, so that the whole slide is equally covered. Some slide types must be pre-cleaned with 70% ethanol to obtain adequate section adherence.
- Start by gradually advancing the face of the resin block towards the knife blade. Do not try sectioning without advancing the block first, or else the equipment and the block can be damaged. If necessary, trim the block using a higher thickness (10 µm and above).
- When approaching a suitable section, make a firm one-way movement with the handwheel, so the section is made at once. Keep a check on the resin moisture. During sectioning, regularly moisturize the face of the block being cut using a paintbrush dipped in distilled water if there is a problem with curling sections. Remove excess water with a tissue paper.
- With a pair of fine point tweezers, place the obtained section in the water over the slide. Upon coming in contact with water, GMA resin stretches. If necessary, use a paintbrush to gently unfold and stretch the sections. Use another paintbrush to constantly keep the blade free of resin debris. Do not switch between the paintbrushes to avoid wetting the blade.
- After queueing up all the desired sections in the slide, dry the bottom face of the slide and place it above the hot plate. Remove the excess water from the top of the slide by gently dabbing with a filter paper (optional). The sections will adhere when the water evaporates from the slide. Do not leave the slides too long to prevent the sections being damaged by excessive heat.
- Store the slides in a slide box, away from dust and the sun, and use them for staining and other procedures. The slides can be stored for several years.
5. Freezing plant samples and sectioning with a cryostat
NOTE: The essential consideration in cryosectioning biological tissue is to reduce damage due to ice crystal formation when freezing samples. Cryoprotection is usually done by infusing chemically inert solutions such as glycerol or sucrose19,20.
- One day before sample sectioning, perform the following steps.
- Dilute 100 mL of 0.2 M PB (Table 3) in 100 mL of distilled water to obtain 200 mL of 0.1 M PB. Prepare 10%, 20%, and 30% sucrose solutions in 0.1 M PB (e.g., for a 10% solution, add 2 g of sucrose in 20 mL of buffer).
- For fresh samples, wash them in 0.1 M PB for 30 min. For samples in a fixative, wash them in the same buffer used for preparing the fixative for 30 min. For samples in 70% ethanol, hydrate them in 50% and 30% ethanol and wash in 0.1 M PB for 1 h in each solution.
- Incubate the samples for 2 h in 10% sucrose, 2 h in 20% sucrose, and 2 h in 30% sucrose, at room temperature. Afterwards, incubate overnight in 30% sucrose at 4 °C (or at least for 3 h; maximum time is 48 h).
- On the day of sectioning, prepare 40% and 50% sucrose in 0.1 M PB; do not prepare sucrose solutions more than 12 h in advance. Incubate for 2 h in each sucrose concentration, at 4 °C.
- For embedding, in small molds, make a layer of OCT compound (optimal cutting temperature medium, used for embedding and freezing the samples) and keep it at -20 °C to freeze. The molds can be regular histological molds, although to facilitate unmolding the blocks, paper or tinfoil ones can be made using a small cuboid as a frame and adhesive tape.
- After the bottom layer of the OCT compound is frozen in the molds, work inside a cryostat chamber (ca. -27 °C). Place the samples incubated in 50% sucrose in the molds, in the orientation in which they will be sectioned. The upper face of a cuboid block is usually a better face of sectioning. Mark in the mold where the samples are placed, so the block can be easily trimmed, and the correct orientation maintained.
- Surround the samples in OCT compound and burst any air bubble touching the samples. Freeze them at -20 °C. With the blocks fully frozen, place them inside the cryostat chamber (ca. -27 °C). Unmold each one only before using and pay attention to the marks that indicate sample location inside the block.
- As OCT compound is easily cut with a blade, appropriately trim the blocks before positioning them onto the chucks. Put some OCT compound in the cryostat chuck and position the block so the upper face is sectioned. Faces with smaller areas provide better sections. Wait until the block is well attached to the chuck and test it before starting to section.
- Place the chuck firmly in the chuck holder. Adjust the orientation of sectioning using the orientation screws. Adjust the angle of the knife using the knife tilt. Select the thickness of the sections. The samples can be sectioned thicker than usual resin sections. Sections in a range between 5-20 µm are successfully obtained, with thicker sections being easier to make (less curling and less damage of structures).
- Advance the face of the frozen block toward the knife blade. Do not try sectioning without doing so, or else the block can be detached from the chuck and damaged. If necessary, trim the block using a higher thickness (10 µm and above).
- When approaching a suitable section, place the anti-roll plate (i.e., a transparent plate that will retain the section) above the knife and make a firm one-way movement with the handwheel, so the section is made at once. Curling problems can be caused if the anti-roll plate needs to be adjusted (it is usually adjustable in reference to the blade) or if there is debris in the blade. Clean the blade constantly with a paintbrush to remove debris.
- Use special slides so the sections attach easily, like silanized slides (commercial or prepared with 2% aminoalkylsilane in acetone21), or slides prepared with 500 µg/mL poly-L-Lysine in distilled water21 or 0.2% gelatin (see details21). Maintain the slides at room temperature.
- To adhere the section to a slide, lift the anti-roll plate and rapidly make the slide touch the section. As the slide is at room temperature, the section immediately melts and adheres to the slide. Pay attention to turn the treated face of the slide to the section, which may remain above the knife or on the inner face of the anti-roll plate. To avoid curling of sections, perform this step rapidly as soon as the plate is lifted and be careful not to contort the section.
- Leave the slide outside the cryostat chamber (at room temperature) if new sections are to be added to it. After adhering all the wanted sections to the slide, keep it inside the cryostat chamber or in the freezer (-20 °C or below). Do not expose the slides to humidity. Keep them in a slide box and remember to identify the slides with a pencil.
NOTE: Slides and blocks of OCT can be stored at -20 °C, although not for too long. To achieve better results, use the slides and the blocks within a few days.
6. Staining plant sections and endophytes for light microscopy
NOTE: Many types of stains can be used for plant sections. It is challenging to differentially stain endophytic fungi and plant tissues. Although not a staining procedure, a method for marking fungi structures is presented in section 7 (fluorescence with a wheat germ agglutinin conjugate). Freehand sections (explained in section 3), resin sections (section 4), and cryosections (section 5) can be stained, although phenol and alcohol-based stains are challenging for these samples as GMA resin and OCT lose adherence to the slide in these cases.
- Use one or combine the following usual staining methods for plant samples.
- Toluidine blue O22,23, a vastly applied method for general staining of plant sections. Prepare a solution of 0.05% toluidine blue O in 0.1 M phosphate (pH 6.8) or 0.09 M citrate buffer (pH 4.5-4.8), depending on the species and types of tissue. Incubate GMA resin sections for 2-10 min using a slide staining jar or by placing some drops above the sections if few slides are stained. Wash carefully with distilled water or buffer after incubation and mount the slides with water or dry them on a hot plate to produce permanent slides as described in step 6.3.
- Lugol reagent2 indicates the presence of starch. Prepare a 5% iodine (I2) and 10% potassium iodide (KI) solution in distilled water. Stain sections for 2 min, by adding few drops above the slide, and then wash with distilled water. This histochemical test is usually applied to temporary slides.
- Sudan III, IV, and black B24,25 stain for different lipids. Prepare a solution of 0.3% Sudan (III, IV, or black B) in 70% ethanol, warm it until boiling, and let cool. Use the supernatant, filter it, and incubate sections for 15-30 min in a closed Petri dish. Wash the sections carefully with 70% ethanol and distilled water. Mount the slides with water (usually applied only to temporary slides).
NOTE: As Sudan is an alcohol-based dye, it is more suitable for freehand sections. Conduct GMA resin section staining carefully as they usually detach from the slide.
- For temporary slides, mount the sections in water or glycerin and observe subsequently. Seal the coverslip with nail polish for preserving them a little longer.
- For permanent slides, mount the sections with synthetic resins (e.g., rapid mounting medium, see Table of Materials). Drip a few drops of the mounting medium (it can overflow the coverslip), put the coverslip carefully to avoid bubbles, and use clothes pegs to press the slide against the coverslip until fully dry. Remove the excess of dried mounting medium with a razor blade.
7. Application of a fluorochrome conjugated to wheat germ agglutinin in fluorescence and confocal microscopy
NOTE: This method can be applied to freehand sections (explained in section 3), resin sections (section 4), and cryosections (section 5). Cryosections can be adequate for confocal microscopy purposes, as thicker samples can be provided when compared to resin sections, but not as thick as freehand ones. A fluorochrome conjugated to wheat germ agglutinin (WGA, see Table of Materials) is applied to fungal imaging in fluorescence microscopy26. A confocal microscope is not essential, although it can provide clear three-dimensional images of plant structures27.
- Prepare a solution of 0.2 mg/mL WGA-fluorochrome conjugate in 0.1 M PB28 (pH 7.2, check step 5.1.1 and Table 3). Prepare a solution of 1% Calcofluor White in 0.1 M PB (pH 7.2). Prepare small amounts of these solutions, as the sections are directly incubated with them.
- Incubate the sections in the glass slides for 30 min in the WGA-fluorochrome conjugate solution29, using enough volume to cover the sections, then wash in 0.1 M PB.
- Incubate the sections in the calcofluor solution, using enough volume as a mounting medium. The solution can be maintained during the observation period.
- Put coverslips on the slides and observe in a confocal microscope or a fluorescence light microscope using the following filters: TC/GFP (excitation: 470-440, emission: 525-550, for WGA-fluorochrome in the Table of Materials - fungal cell wall fluoresces green under this filter29) and DAPI (excitation: 358, emission: 463, for Calcofluor White)30.
NOTE: Three-dimensional images can be obtained using the Z-series function in the confocal microscope27.
8. Scanning electron microscopy of plant organs
- After fixing samples, performing dehydration, and storing in 70% ethanol (section 1), one possibility is to cut samples to expose any desired surface for SEM analysis, if necessary (e.g., internal tissues, ovary structure). Use a sharp and new razor blade and make cuts with a one-way movement, avoiding a damaged appearance of these areas in SEM. If necessary, use a stereomicroscope to select the samples and consider the metal stubs area to determine sample sizes.
- Further dehydrate samples for SEM in an ethanolic series: 80%, 96%, and 2x in absolute ethyl alcohol (≥99.8%). Maintain small and delicate samples for 30 min in each concentration and larger and denser samples for 1 h.
- Fold small envelopes using tissue paper to organize samples for the next steps, larger samples can be handled without an envelope. Identify the envelopes with a pencil by writing a letter or a number, and keep a log of all the samples in each one. Keep the samples in absolute ethanol, although not for long, and proceed to step 8.4 as soon as possible.
- Proceed for critical-point (CP) drying. Operate a CP dryer according to standard operating procedures. Place samples in absolute ethanol (intermediate fluid) in a pressure chamber. At the CO2 critical point (31 °C, 7.3 x 106 Pa) the intermediate fluid dissolves into the transition fluid (liquid carbon dioxide), and the samples are dried31.
- After CP drying, store samples as soon as possible in a desiccation container, for instance, a sealed flask containing silica gel. Atmospheric humidity can destroy the samples if reabsorbed31.
- Use metal stubs to mount the samples. Before mounting, put gloves on to manipulate the stubs, immerse them in acetone for 5 min to eliminate any fat, and let them dry. Use a conductive double-sided carbon adhesive tape to fix samples on the stub, and a stereomicroscope to help position samples, keeping in mind that the sight from above is the only possible perspective in SEM images.
- Manipulate samples with fine-point tweezers, being careful as the sample part touched by the tweezers is usually damaged, so try touching parts positioned away from the areas of interest (e.g., areas in contact with the tape). Maintain the stubs with samples in a sealed Petri dish with silica gel. Proceed to step 8.8 as soon as possible.
- Use a sputter coater to deposit a layer of metal, usually gold or platinum, on the surface of the samples in a low-pressure atmosphere of an inert gas, frequently argon31. Follow standard operating procedures when using a sputter coater. The coating thickness depends on the topography of the samples, usually between 15-40 nm32.
- Maintain the coated stubs in a sealed Petri dish with silica gel, and provided that the silica gel is retaining humidity, samples can be stored this way for weeks. Use a scanning electron microscope to analyze the samples. The samples in vacuo are struck by a beam of electrons, and the emission of signals from such interaction is interpreted as images31. For details on operating a scanning electron microscope, read Jeffree and Read (1991)31 and Bozzola and Russell (1999)32.
- To reuse the stubs, pull the adhesive tape and scrub them with wire wool. Wash in tap water, immerse in absolute ethanol, and dry them adequately, preventing oxidation of the constituent metal.
9. Transmission electron microscopy
- Prefix samples with glutaraldehyde-cacodylate buffer as explained in steps 1.7 and 1.10. After 12-24 h of prefixation, wash the samples 3x in 0.2 M cacodylate buffer (pH 7.25) for 10 min. Perform postfixation with 1% osmium tetroxide (OsO4) in 0.2 M cacodylate buffer, for 12 h in the dark, at room temperature. Wash 3x with distilled water for 5 min.
CAUTION: Cacodylate and osmium tetroxide are highly toxic and should not be inhaled. Use them in fume hoods, following the respective safety data sheets. - Dehydrate the samples with 30%, 50%, 70%, and 96% ethanol, 2x in each concentration, for 10 min. Then, dehydrate 3x in absolute alcohol, for 15 min each time.
- Infiltrate the samples in hydrophilic acrylic resins (see Table of Materials), once with 1:1 resin + absolute ethanol and 3x with pure resin for 8-12 h each. Conduct polymerization in gelatin capsules at 60 °C until completely solidified (12 h maximum17).
- Closely evaluate the orientation of samples inside the resin block; cut the top part of the block, with a razor blade, making a pyramidal shape that concentrates the sample in the sectioning area. Obtain semithin sections (250-500 nm)33 in an ultramicrotome with a diamond knife and place on glass slides in few drops of water.
- Keep the slides on a hot plate at 60 °C. Stain the sections with toluidine blue O as in step 6.1.1 and let the stain dry completely. Wash carefully with tap water. Evaluate the obtained section by drawing four quadrants and selecting the most suitable quadrant for analysis.
- Trim the block so the pyramidal shape concentrates the chosen quadrant on the block's upper face. Produce ultrathin sections (50-100 nm)33,34. Thickness is evaluated according to the interference color of the sections: sections with about 70 nm appear silver-gold, with about 100 nm appear gold, and with about 200 nm appear blue34.
- Collect the ultrathin sections from water using copper grids and proceed to the contrast staining method with uranyl acetate and lead citrate, as described below.
- Prepare a lead citrate solution (Table 5) and freeze the final solution in microcentrifuge tubes with 1 mL of solution in each, only thawing right before use.
- Prepare a uranyl acetate solution: dissolve 0.625 g of uranyl acetate [UO2(CH3COO)2] in 25 mL of recently boiled and cooled distilled water. Store in a dark flask in the freezer.
CAUTION: Lead nitrate is toxic if ingested. 1 N NaOH is highly corrosive. Uranyl acetate is radioactive and toxic. It must not be ingested, inhaled, or come in contact with the skin. - When staining, put both prepared reagents in separate 3 mL syringes with filter units (0.22 µm pore, see Table of Materials). Prepare a Petri dish turned upside-down with a sealing thermoplastic film over it (see Table of Materials) and inside a wider dish, with NaOH pellets on the edges as a trap for CO232 (see Figure 2).
- Discard the first drop, and over the film place a drop of uranyl acetate and three drops of distilled water for each grid stained. Do the same with lead citrate and add three more drops of distilled water.
- Incubate the grid (with the opaque side downwards, where the sections are) in uranyl for 30 min (variable time). Wash 3x in the distilled water drops, drying the grid each time gently with filter paper on the brilliant side. Repeat with the lead citrate drop (30 min) and wash it.
- After at least 4 h, analyze the grids in a transmission electron microscope. In this microscope, a beam of electrons passes through the sections in vacuo and the image is projected on a fluorescent screen. For details on operating a transmission electron microscope, read Bozzola and Russell (1999)32.
| lead citrate solution (for TEM contrast staining) | |
| step 1 | surround a beaker with tinfoil |
| step 2 | dissolve 0.266 g of lead nitrate [Pb(NO3)2] in 6 mL of recently boiled and cooled distilled water |
| step 3 | agitate for 2 min |
| step 4 | add 0.352 g of trisodium citrate [Na3(C6H5O7).2H2O] (the solution must acquire a milky appearance) |
| step 5 | agitate for 15 min, seal the beaker with tinfoil and transfer the solution to a 10 mL beaker |
| step 6 | add 1.6 mL of 1N NaOH and 2.4 mL of distilled water (the solution must be translucent) |
| step 7 | if necessary, adjust the pH close to 12 |
Table 5: Lead citrate solution recipe.

Figure 2: Contrast staining scheme with lead citrate and uranyl acetate solutions. (A) Prepare the Petri dishes, one turned upside-down (in the center) with thermoplastic film so drops can be placed above it, inside a wider one. NaOH pellets are places around the central dish. (B) Uranyl acetate drops are placed in the circles with the letter U, and lead citrate drops in the circles marked L. DW indicates drops of distilled water. The grids are stained sequentially in the column, so five grids can be stained simultaneously as represented. Please click here to view a larger version of this figure.
10. Symbiotic germination of orchid seeds
- Ensure that the solutions and all the materials used in symbiotic germination of seeds are sterile to avoid contamination. Start by autoclaving them for 20 min at 121 °C. The symbiotic germination steps are summarized in Figure 3.
- Superficially disinfect fruits and seeds by immersing them in sodium hypochlorite solution containing 2% active chlorine for 10-15 min for fruits and 7-10 min for seeds, considering the stiffness and thickness of seed coat9. Slim and fragile seeds can be immersed in a 1:1 diluted sodium hypochlorite solution. Afterwards, wash 3x in autoclaved distilled water to remove the hypochlorite solution.
- Recuperate the seeds by filtering in serigraphic fabric and use the seeds to proceed with germination tests (preferably). If necessary, store them in filter paper envelopes inside glass flasks with silica gel, at 4 °C, hermetically close the flasks and seal them with cling film. Transfer some drops of water from the last wash to potato dextrose agar (PDA, 39 g/L), to evaluate the effectiveness of the washing process.
- Before sowing seeds in the culture medium, evaluate their viability through the tetrazolium test (optional) as described below35.
- Incubate approximately 10 mg of seeds in a microcentrifuge tube with 1 mL of 10% sucrose in distilled water, for 24 h at room temperature (ca. 25 °C), in light.
- Remove the sucrose solution with a micropipette and add 1 mL of 1% tetrazolium solution (triphenyltetrazolium chloride) in distilled water. Incubate at 40 °C in a thermoblock for 24 h in the dark.
- Remove the tetrazolium solution with a micropipette and wash the seeds with distilled water 2x or until the solution is removed. Remove all liquids. If necessary, the seeds can be stored in the freezer for up to a week (as in step 10.3) before being analyzed.
- Resuspend the seeds in distilled water and analyze them under a light microscope. Viable seeds acquire light to dark red color, while non-viable seeds retain their natural color.
- Perform the following adapted9 protocol for symbiotic germination of orchid seeds.
- Incubate the seeds over autoclaved filter paper discs (1-2 cm in diameter) placed in Petri dishes with oatmeal agar (OMA) culture medium (2.5 g/L of oat flakes and 7 g/L of agar, pH 6).
- In the center of the Petri dish, inoculate a fragment of culture medium (ca. 1 cm2) containing mycelium from the chosen isolated fungus for germination procedure. Seal the Petri dishes with cling film and incubate them in the dark at around 25 °C or room temperature, as it is more adequate for fungal growth.
- Prepare some dishes with seeds and without fungal inoculation, as a negative control for the germination test.
- Analyze the germination results weekly, by collecting quantitative and qualitative data and photographing protocorms and seedlings. The observation of seeds and protocorms should be performed using a stereomicroscope for a more accurate assessment of germination. Use a light source coming from below, as it allows for greater contrast, making it possible to discriminate the fungal mycelium from protocorms more easily.
- Collect samples in different developmental stages and fix for anatomical analyses (section 1). Apply all the image analyses previously described to investigate fungal endophytes in seeds, protocorms, and seedlings during germination (light microscopy - sections 4, 5, and 6; confocal and fluorescence - section 7; SEM and TEM - sections 8 and 9).
- Generate quantitative results following the classification of stages according to Table 6. The stages describe the usual development of seeds from mycoheterotrophic orchids. Collect data weekly and table with the initial dates of each observed stage.
- Additionally, collect quantitative data estimating germination percentage and rate. Count at least 100 seeds or define counting fields35. Demarcate three or more counting fields per Petri dish, consisting of fixed regions with a standardized area, and evaluate weekly. Calculate collected data according to the growth index (GI) equation:

where N0 is the number of counted seeds in stage 0, N1 refers to stage 1, and it follows until stage 6 (registered as N6)36.
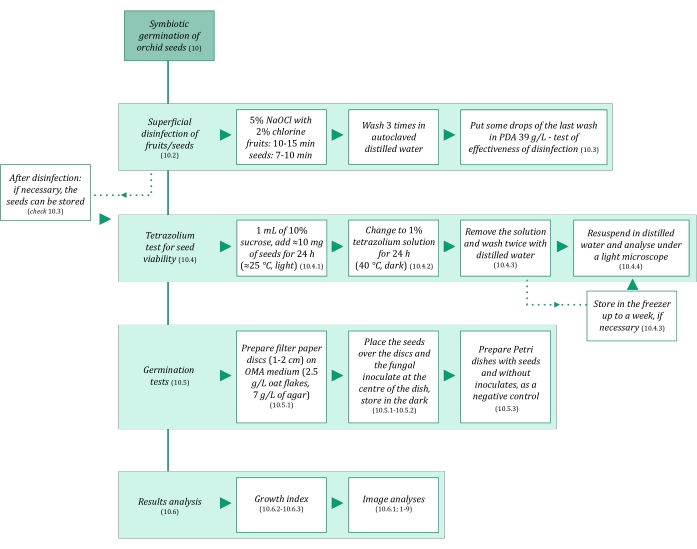
Figure 3: Schematic summarization of symbiotic germination of seeds methodology. The schematics provide indications of detailed steps in the protocol. Abbreviations: OMA = oatmeal agar, PDA = potato dextrose agar. Please click here to view a larger version of this figure.
| Germination stage | Description |
| 0 | No germination |
| 1 | Swelling of the embryo |
| 2 | Testa rupture |
| 3 | Absorbent hairs develop |
| 4 | Stem projection develops |
| 5 | Protecting scales (bracts) develop |
| 6 | First roots develop |
Table 6: Description of protocorm developmental stages applied to periodic analyses of germination tests. Modified from stages described in Otero et al.36.
Access restricted. Please log in or start a trial to view this content.
Results
Following the essential stages of fixing plant tissue yields cellular structures as similar as possible to the living state, considering the morphology, volume, and spatial organization of cellular components and tissues16. Observe such traits in the samples after chemical fixation (Figure 4). Figure 4C-F represents adequately fixed samples under light microscopy. Following the fixation procedures de...
Access restricted. Please log in or start a trial to view this content.
Discussion
Image analyses in plant anatomy and morphology have an important potential to fulfil objectives and help understand the relationships between mycoheterotrophic plants and their indispensable fungal endophytes, as demonstrated by studies of subterranean organs6,40, structural analyses of symbiotic germination of seeds39, and aerial and reproductive structures41. Structural botany, despite having lost its prestige and...
Access restricted. Please log in or start a trial to view this content.
Disclosures
The authors have nothing to disclose.
Acknowledgements
The authors thank funding from FAEPEX and FAPESP (2015/26479-6). MPP thanks Capes for his master's degree scholarship (process 88887.600591/2021-00) and CNPq. JLSM thanks CNPq for productivity grants (303664/2020-7). The authors also thank the access to equipment and assistance provided by LME (Laboratory of Electron Microscopy - IB/Unicamp), INFABiC (National Institute of Science and Technology on Photonics Applied to Cell Biology - Unicamp), and LaBiVasc (Laboratory of Vascular Biology - DBEF/IB/Unicamp); LAMEB (UFSC) and Eliana de Medeiros Oliveira (UFSC) for contributions to cryoprotection protocol; LME for contributions to TEM protocol.
Access restricted. Please log in or start a trial to view this content.
Materials
| Name | Company | Catalog Number | Comments |
| Acetone | Sigma-Aldrich | 179124 | (for SEM stubs mounting) |
| Agar-agar (AA) | Sigma-Aldrich | A1296 | (for seeds germination tests) |
| Calcofluor White Stain | Sigma-Aldrich | 18909 | fluorescent dye (detects cellulose) |
| Citrate Buffer Solution, 0.09M pH 4.8 | Sigma-Aldrich | C2488 | (for toluidine blue O staining) |
| Conductive Double-Sided Carbon Tape | Fisher Scientific | 50-285-81 | (for SEM) |
| Confocal Microscope | Zeiss | (any model) | |
| Copper Grids | Sigma-Aldrich | G4776 | (for TEM) |
| Critical-point dryer | Balzers | (any model) | |
| Cryostat | Leica Biosystems | (any model) | |
| Dissecting microscope | Leica Biosystems | (= stereomicroscope, any model) | |
| Entellan | Sigma-Aldrich | 107960 | rapid mounting medium for microscopy |
| Ethyl alcohol, pure (≥99.5%) | Sigma-Aldrich | 459836 | (= ethanol, for dehydration processes) |
| Formaldehyde solution, 37% | Sigma-Aldrich | 252549 | (for NBF solution preparation) |
| Formalin solution, neutral buffered, 10% | Sigma-Aldrich | HT501128 | histological tissue fixative |
| Gelatin capsules for TEM | Fisher Scientific | 50-248-71 | (for resin polymerisation in TEM) |
| Gelatin solution, 2% in H2O | Sigma-Aldrich | G1393 | (dilute for slides preparation - OCT adherence) |
| Glutaraldehyde solution, 25% | Sigma-Aldrich | G6257 | (for Karnovsky’s solution preparation) |
| HistoResin | Leica Biosystems | 14702231731 | glycol methacrylate (GMA) embedding kit |
| Iodine | Sigma-Aldrich | 207772 | (for Lugol solution preparation) |
| Lead(II) nitrate | Sigma-Aldrich | 228621 | Pb(NO3)2 (for TEM contrast staining) |
| Light Microscope | Olympus | (any model) | |
| LR White acrylic resin | Sigma-Aldrich | L9774 | hydrophilic acrylic resin for TEM |
| Lugol solution | Sigma-Aldrich | 62650 | (for staining) |
| Metal stubs for specimen mounts | Rave Scientific | (for SEM, different models) | |
| Microtome | Leica Biosystems | manual rotary microtome or other model | |
| Oatmeal agar (OMA) | Millipore | O3506 | (for seeds germination tests) |
| OCT Compound, Tissue-Tek | Sakura Finetek USA | 4583 | embedding medium for frozen tissues |
| Osmium tetroxide | Sigma-Aldrich | 201030 | OsO4 (for TEM postfixation) |
| Parafilm M | Sigma-Aldrich | P7793 | sealing thermoplastic film |
| Paraformaldehyde | Sigma-Aldrich | 158127 | (for Karnovsky’s solution preparation) |
| Poly-L-lysine solution, 0.1% in H2O | Sigma-Aldrich | P8920 | (for slides preparation - OCT adherence) |
| Poly-Prep Slides | Sigma-Aldrich | P0425 | poly-L-lysine coated glass slides |
| Polyethylene Molding Cup Trays | Polysciences | 17177A-3 | (6x8x5 mm, for embbeding samples in GMA resin) |
| Polyethylene Molding Cup Trays | Polysciences | 17177C-3 | (13x19x5 mm, for embbeding samples in GMA resin) |
| Potassium iodide | Sigma-Aldrich | 221945 | (for Lugol solution preparation) |
| Potato Dextrose Agar (PDA) | Millipore | 70139 | (for seeds germination tests) |
| Scanning Electron Microscope | Jeol | (any model) | |
| Silane [(3-Aminopropyl)triethoxysilane] | Sigma-Aldrich | A3648 | (for slides preparation - OCT adherence) |
| Silane-Prep Slides | Sigma-Aldrich | S4651 | glass slides coated with silane |
| Silica gel orange, granular | Supelco | 10087 | (for dessicating processes) |
| Sodium cacodylate trihydrate | Sigma-Aldrich | C0250 | (for glutaraldehyde-sodium cacodylate buffer) |
| Sodium hydroxide | Sigma-Aldrich | S5881 | NaOH (for Karnovsky’s solution preparation and TEM contrast staining) |
| Sodium hypochlorite solution | Sigma-Aldrich | 425044 | NaClO (for seeds surface disinfection) |
| Sodium phosphate dibasic, anhydrous | Sigma-Aldrich | 71640 | Na2HPO4 (for NBF solution and PB preparation) |
| Sodium phosphate monobasic monohydrate | Sigma-Aldrich | S9638 | NaH2PO4·H2O (for NBF and PB) |
| Sputter coater | Balzers | (any model) | |
| Sucrose | Sigma-Aldrich | S0389 | C12H22O11 (for cryoprotection and germination test) |
| Sudan III | Sigma-Aldrich | S4131 | (for staining) |
| Sudan IV | Sigma-Aldrich | 198102 | (for staining) |
| Sudan Black B | Sigma-Aldrich | 199664 | (for staining) |
| Syringe | (3 mL, any brand, for TEM contrast staining) | ||
| Syringe Filter Unit, Millex-GV 0.22 µm | Millipore | SLGV033R | PVDF, 33 mm, gamma sterilized (for TEM contrast staining) |
| Tek Bond Super Glue 793 | Tek Bond Saint-Gobain | 78072720018 | liquid cyanoacrylate adhesive, medium viscosity |
| Toluidine Blue O | Sigma-Aldrich | T3260 | (for staining) |
| Transmission Electron Microscope | Jeol | (any model) | |
| Triphenyltetrazolium chloride | Sigma-Aldrich | T8877 | (for the tetrazolium test in seeds germination) |
| Trisodium citrate dihydrate | Sigma-Aldrich | S1804 | Na3(C6H5O7)·2H2O (for TEM contrast staining) |
| Ultramicrotome | Leica Biosystems | (any model) | |
| Uranyl acetate | Fisher Scientific | 18-607-645 | UO2(CH3COO)2 (for TEM contrast staining) |
| Vacuum pump | (any model) | ||
| Wheat Germ Agglutinin, Alexa Fluor 488 Conjugate | TermoFisher Scientific | W11261 | fluorescent dye-conjugated lectin (detects sialic acid and N-acetylglucosaminyl residues) |
References
- Evert, R. F. Esau’s Plant Anatomy: Meristems, Cells, and Tissues of the Plant Body: Their Structure, Function, and Development. , John Wiley & Sons. Hoboken, NJ, USA. (2006).
- Yeung, E. C. T., Stasolla, C., Sumner, M. J., Huang, B. Q. Plant Microtechniques and Protocols. , Springer International Publishing. Cham, Switzerland. (2015).
- Sokoloff, D. D., Jura-Morawiec, J., Zoric, L., Fay, M. F. Plant anatomy: at the heart of modern botany. Botanical Journal of the Linnean Society. 195 (3), 249-253 (2021).
- Leake, J. R. The biology of myco-heterotrophic (‘saprophytic’) plants. New Phytologist. 127 (2), 171-216 (1994).
- Bidartondo, M. I. The evolutionary ecology of myco-heterotrophy. New Phytologist. 167 (2), 335-352 (2005).
- Imhof, S., Massicotte, H. B., Melville, L. H., Peterson, R. L. Subterranean morphology and mycorrhizal structures. Mycoheterotrophy. , Springer. New York, NY. 157-214 (2013).
- Rasmussen, H. N., Rasmussen, F. N. Orchid mycorrhiza: implications of a mycophagous life style. Oikos. 118 (3), 334-345 (2009).
- Rasmussen, H. N., Dixon, K. W., Jersáková, J., Těšitelová, T. Germination and seedling establishment in orchids: a complex of requirements. Annals of Botany. 116 (3), 391-402 (2015).
- Zettler, L. W. Terrestrial orchid conservation by symbiotic seed germination: techniques and perspectives. Selbyana. 18 (2), 188-194 (1997).
- Stewart, S. L., Kane, M. E. Symbiotic seed germination and evidence for in vitro mycobiont specificity in Spiranthes brevilabris (Orchidaceae) and its implications for species-level conservation. In Vitro Cellular & Developmental Biology - Plant. 43 (3), 178-186 (2007).
- Zhao, D. -K., et al. Orchid reintroduction based on seed germination-promoting mycorrhizal fungi derived from protocorms or seedlings. Frontiers in Plant Science. 12, 701152(2021).
- Selosse, M. A., Roy, M. Green plants that feed on fungi: facts and questions about mixotrophy. Trends in Plant Science. 14 (2), 64-70 (2009).
- Merckx, V. S. F. T., Mennes, C. B., Peay, K. G., Geml, J. Evolution and diversification. Mycoheterotrophy: The Biology of Plants Living on Fungi. , Springer. New York, NY. 215-244 (2013).
- Boon, M. E., Drijver, J. Routine Cytological Staining Techniques: Theoretical Background and Practice. Macmillan International Higher Education. , Hampshire, UK. (1986).
- Karnovsky, M. A formaldehyde-glutaraldehyde fixative of high osmolality for use in electron microscopy. Journal of Cell Biology. 27 (2), 137-138 (1964).
- Hayat, M. Fixation for Electron Microscopy. , Academic Press. New York, USA. (1981).
- Roland, J. C., Vian, B. General preparation and staining of thin sections. Electron Microscopy of Plant Cells. 1, 675(1991).
- Gerrits, P. O., Horobin, R. W. Glycol methacrylate embedding for light microscopy: basic principles and trouble-shooting. Journal of Histotechnology. 19 (4), 297-311 (1996).
- Zhang, Z., Niu, L., Chen, X., Xu, X., Ru, Z. Improvement of plant cryosection. Frontiers in Biology. 7 (4), 374-377 (2012).
- BeneŠ, K. On the media improving freeze-sectioning of plant material. Biologia Plantarum. 15 (1), 50-56 (1973).
- Fischer, A. H., Jacobson, K. A., Rose, J., Zeller, R. Preparation of slides and coverslips for microscopy. Cold Spring Harbor Protocols. 2008 (5), (2008).
- Sakai, W. S. Simple method for differential staining of paraffin embedded plant material using toluidine blue O. Stain Technology. 48 (5), 247-249 (1973).
- O’Brien, T., Feder, N., McCully, M. E. Polychromatic staining of plant cell walls by toluidine blue O. Protoplasma. 59 (2), 368-373 (1964).
- Ventrella, M. C., Almeida, A. L., Nery, L. A., Coelho, V. P. deM. Métodos Histoquímicos Aplicados às Sementes. Universidade Federal de Viçosa. , Viçosa, Brazil. (2013).
- Pearse, A. G. E. Histochemistry, Theoretical and Applied. , J & A Churchill. London, UK. (1960).
- Andrade-Linares, D. R., Franken, P. Fungal endophytes in plant roots: taxonomy, colonization patterns, and functions. Symbiotic Endophytes. , Springer. Berlin. 311-334 (2013).
- Wymer, C. L., Beven, A. F., Boudonck, K., Lloyd, C. W. Confocal microscopy of plant cells. Confocal Microscopy Methods and Protocols. , Humana Press. Totowa. 103-130 (1999).
- Marques, J. P. R., Soares, M. K. M. Manual de Técnicas Aplicadas à Histopatologia Vegetal. FEALQ. , Piracicaba, Brazil. (2021).
- Navarro, B. L., Marques, J. P. R., Appezzato-da-Glória, B., Spósito, M. B. Histopathology of Phakopsora euvitis on Vitis vinifera. European Journal of Plant Pathology. 154 (4), 1185-1193 (2019).
- Marques, J. P. R., et al. Sugarcane cell wall-associated defense responses to infection by Sporisorium scitamineum. Frontiers in Plant Science. 9, 698(2018).
- Jeffree, C. E., Read, N. D. Ambient-and low-temperature scanning electron microscopy. Electron Microscopy of Plant Cells. , 313-413 (1991).
- Bozzola, J. J., Russell, L. D. Electron Microscopy: Principles and Techniques for Biologists. , Jones & Bartlett Learning. Sudbury, MA, USA. (1999).
- Murray, S. Basic transmission and scanning electron microscopy. Introduction to electron Microscopy for Biologists. , Academic Press. 3-18 (2008).
- Tanaka, M. Glossary of TEM terms. , Available from: https://www.jeol.co.jp/en/words/emterms/ (2021).
- Seaton, P. T., et al. Orchid seed and pollen: a toolkit for long-term storage, viability assessment and conservation. Orchid Propagation: From Laboratories to Greenhouses—Methods and Protocols. , Humana Press. New York, NY. 71-98 (2018).
- Otero, J. T., Ackerman, J. D., Bayman, P. Differences in mycorrhizal preferences between two tropical orchids. Molecular Ecology. 13 (8), 2393-2404 (2004).
- Koch, R. A., et al. Marasmioid rhizomorphs in bird nests: Species diversity, functional specificity, and new species from the tropics. Mycologia. 112 (6), 1086-1103 (2020).
- Webster, J., Weber, R. Introduction to Fungi. , Cambridge University Press. Cambridge, UK. (2007).
- Sisti, L. S., et al. The role of non-mycorrhizal fungi in germination of the mycoheterotrophic orchid Pogoniopsis schenckii Cogn. Frontiers in Plant Science. 10, 1589(2019).
- Martos, F., et al. Independent recruitment of saprotrophic fungi as mycorrhizal partners by tropical achlorophyllous orchids. New Phytologist. 184 (3), 668-681 (2009).
- Alves, M. F., et al. Reproductive development and genetic structure of the mycoheterotrophic orchid Pogoniopsis schenckii Cogn. BMC Plant Biology. 21 (1), 332(2021).
- Merckx, V. S. F. T. Mycoheterotrophy: an introduction. Mycoheterotrophy: The Biology of Plants Living on Fungi. , 1-17 (2013).
- Hall, J. L., Hawes, C. Electron Microscopy of Plant Cells. , Academic Press. Cambridge, UK. (1991).
Access restricted. Please log in or start a trial to view this content.
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved