A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Detached Maize Sheaths for Live-Cell Imaging of Infection by Fungal Foliar Maize Pathogens
In This Article
Summary
This manuscript details an optimized inoculation protocol that uses detached maize leaf sheaths for reproducible cytological, physiological, and molecular studies of maize interactions with fungal plant pathogens. The leaf sheaths facilitate real-time observation of cellular interactions between the living plant and fungus in unfixed tissues.
Abstract
We have optimized a protocol to inoculate maize leaf sheaths with hemibiotrophic and necrotrophic foliar pathogenic fungi. The method is modified from one originally applied to rice leaf sheaths and allows direct microscopic observation of fungal growth and development in living plant cells. Leaf sheaths collected from maize seedlings with two fully emerged leaf collars are inoculated with 20 µL drops of 5 x 105 spores/mL fungal spore suspensions and incubated in humidity chambers at 23 °C under continuous fluorescent light. After 24-72 h, excess tissue is removed with a razor blade to leave a single layer of epidermal cells, an optically clear sample that can be imaged directly without the necessity for chemical fixation or clearing. Plant and fungal cells remain alive for the duration of the experiment and interactions can be visualized in real-time. Sheaths can be stained or subjected to plasmolysis to study the developmental cytology and viability of host and pathogen cells during infection and colonization. Fungal strains transformed to express fluorescent proteins can be inoculated or co-inoculated on the sheaths for increased resolution and to facilitate the evaluation of competitive or synergistic interactions. Fungal strains expressing fluorescent fusion proteins can be used to track and quantify the production and targeting of these individual proteins in planta. Inoculated sheath tissues can be extracted to characterize nucleic acids, proteins, or metabolites. The use of these sheath assays has greatly advanced the detailed studies of the mechanisms of fungal pathogenicity in maize and also of fungal protein effectors and secondary metabolites contributing to pathogenicity.
Introduction
Spatial and temporal analyses at the cellular level are critical for understanding the physiology and cytology of fungal-plant interactions. Foliar tissues that have been chemically fixed1,2,3or cleared and stained4, as well as artificial membranes5, have been used in the past to investigate the cytology of foliar pathogen development and plant-fungal interactions. However, investigation of infection events in living host tissues in real-time without fixation or clearing is challenging due to technical issues related to the preparation of optically transparent samples for imaging.
A detached leaf sheath inoculation protocol was developed in the late 1940s for bright field microscopic investigation of resistance of living rice epidermal cells to the rice blast fungus Magnaporthe oryza6. More recently, detailed molecular, physiological, and cytological observations of host colonization by Colletotrichum and Magnaporthe species have been greatly facilitated by combining modified versions of this leaf sheath method with fungal transformants expressing fluorescent proteins, and high-performance live-cell imaging protocols, including epifluorescence and confocal microscopy7,8,9,10,11,12,13.
This paper details an optimized inoculation protocol using detached maize leaf sheaths for observation of infection processes by hemibiotrophic and necrotrophic foliar fungal pathogens. We have specifically used it to study Colletotrichum graminicola (C. graminicola), the causal agent of anthracnose leaf blight and stalk rot, and Stenocarpella maydis, which causes Diplodia leaf blight and stalk rot. However, the method should be applicable to other hemibiotrophic and necrotrophic foliar fungal pathogens. Cytological and physiological responses during infection and colonization events in these excised leaf sheaths are similar to those in entire leaf blades12,14,15. Furthermore, hemibiotrophic colonization of sheath epidermal cells by C. graminicola is similar to colonization of stalk pith cells16,17. Detached sheaths show greater synchronicity and experimental reproducibility of fungal penetration and colonization than leaf blades or stalk pith tissues14,16,17,18. Most maize varieties can be used for this protocol. However, inbreds or hybrids with excessive purple pigments in the sheaths are less suitable since the pigments interfere with imaging. Golden Jubilee sweet corn has been particularly useful for our studies because untreated seeds are commercially available, the plants are highly susceptible to many foliar diseases, and they grow well in the greenhouse. The first epidemics of anthracnose stalk rot in the United States resulted in the total loss of sweet corn crops in Indiana in the 1970s19,20. This leaf sheath inoculation method can be applied to directly observe and quantify fungal growth and development in living vs. locally killed plant cells, to demonstrate resistance reactions in compatible/incompatible responses to fungal infection, and to test interactions between fungal strains on the same sheath in real-time.
Protocol
NOTE: The workflow for the method is shown in Figure 1.
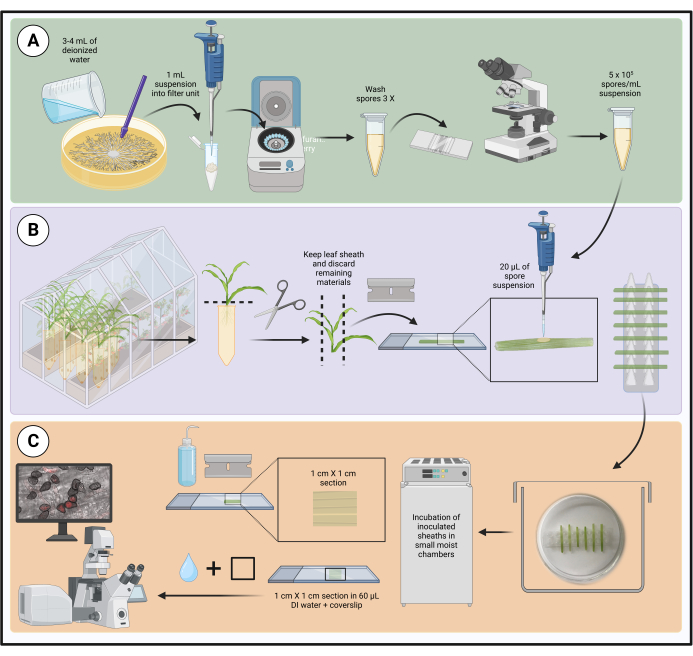
Figure 1: Steps in the optimized inoculation protocol using detached maize leaf sheaths. Spore suspension preparation, leaf sheath inoculation, and sample preparation for live-cell microscopy are highlighted in green (A), purple (B), and orange (C) boxes, respectively. Created with BioRender.com. Please click here to view a larger version of this figure.
1. Plant and fungal material
- Plant growth
- Grow maize seedlings (see Table of Materials) in the greenhouse (14 h light/27 °C and 10 h dark/22 °C) in SC10 containers (see Table of Materials). Use a growth medium consisting of three parts commercial potting soil (see Table of Materials) and two parts steam-sterilized topsoil.
- Water seedlings for 2 min with an overhead irrigation system once every day, in the morning.
- Harvest seedlings at the V2 stage by cutting them at the soil level. The V2 growth stage is reached when seedlings are 5 to 10 cm tall and have two visible collared leaves21.
- Wrap the plants with a damp paper towel and place them in a plastic bag for transport to the laboratory for further processing.
- Fungal cultures
- Reactivate stored fungal silica stock cultures under aseptic conditions22by sprinkling approximately 50 granules of the infested silica on an appropriate agar medium. To avoid morphological changes and loss of pathogenicity, subculture strains no more than 3x after reactivation. Potato dextrose agar (PDA; see Table of Materials) is routinely used to culture C. graminicola while oatmeal agar (OA; see Table of Materials) is used for S. maydis.
- To prepare PDA for culturing fungal stocks, suspend 19.5 g of commercially prepared dehydrated PDA in 500 mL of deionized (DI) water. To make OA, boil 36 g of commercially prepared dehydrated OA in DI water for 15-30 min, filter through three layers of cheesecloth, and bring filtrate to 500 mL with DI water. Autoclave media to sterilize.
- Augment the molten, cooled media with an appropriate antibiotic (e.g., hygromycin B, geneticin) when working with transformant strains. The final concentrations of hygromycin or geneticin in 500 mL of media are 250 µg/mL and 100 µg/mL, respectively.
- Incubate cultures under optimal conditions until a sporulating mycelium has developed. Colletotrichum graminicola and S. maydis grow well at 23 °C with continuous fluorescent light and ambient humidity levels. Sporulation occurs by 7 days after inoculation (dai) for S. maydis or 14 dai for C. graminicola.
2. Leaf sheath inoculations
- Glass-wool filter unit assembly
- Use heavy-duty scissors or shears to cut off the cap and approximately 0.5 mm from the conical bottom of a 1.5 mL microcentrifuge tube (see Table of Materials).
- Place a piece of glass wool (approximately 0.5 cm x 0.5 cm; see Table of Materials) inside the cut tube to cover the center hole, as depicted in Figure 2.
- Wear gloves while cutting glass wool, as borosilicate glass can cause irritation.
- Preparation of leaf sheath support racks
- Cut a non-skirted 96-well PCR plate (see the Table of Materials) into six two-column (8 x 2 wells) support racks as depicted in Figure 3A.
- Flip the two-column racks so the conical bottoms of the wells face up and the flat tops face down (Figure 3B).
- Place each support in a glass Petri plate containing moistened filter paper and cover it with the lid (see Table of Materials). This unit functions as a small humidity chamber for the sheaths.
- Inoculum preparation
- For each fungal strain, place one assembled glass-wool filter unit into an intact sterile 1.5 mL microcentrifuge tube as depicted in Figure 2. The latter functions as a collection tube.
- Label the tubes.
- Harvest conidia from sporulating cultures by first flooding each plate with 3-4 mL of sterile DI water. More water may be necessary in some cases to produce a layer of liquid on the colony surface. Wetting agents are not necessary in this system.
- Loosen the spores from the agar by using a sterile conical-tip pestle (see Table of Materials) to scrape evenly across the entire plate.
- Apply 1 mL of the spore suspension to a glass wool filter unit aseptically and allow the spores to flow through into the collection tube by gravity.
- Centrifuge the collection tubes containing the filtered spore suspensions at 3,500 x g for 5 min. The spores should be pelleted at the bottom of the microcentrifuge tube.
NOTE: Centrifuging C. graminicola spores at higher speeds than recommended here results in a significant loss of viability. - Pour off the liquid into an autoclavable container, add 1 mL of sterile DI water, and gently agitate to resuspend the pelleted spores. Centrifuge as in step 2.3.6.
- Wash spores 3x to remove any conidial matrix that may contain auto inhibitors that might reduce germination or penetration.
- After the third wash, add 300-500 µL of sterile DI water to resuspend spores for quantification.
- Use a hemocytometer under a compound microscope at 100x magnification to determine the spore concentration. Spore staining is not necessary before counting.
- Prepare a 5 x 105 spores/mL suspension with sterile DI water.
NOTE: C. graminicola spore suspension can be kept at room temperature for no longer than 4 h before rapid loss of viability. Refrigerating conidia does not increase viability.
- Sheath inoculations
- Check relevant biosafety practices and procedures before inoculating plants with fungal strains.
- Remove the sheath from the first true leaf of V2 seedlings by running a thumbnail along the overlapping margin of the sheath, and gently loosen it from the shoot. Loosen the sheath from both sides of the shoot before trying to remove it.
- Cut the recovered leaf sheaths into 3-5 cm segments. It is not necessary to surface disinfect the sheaths before inoculation.
- Unroll each segment very gently to expose the inner (adaxial) epidermal layer.
- While preparing sheaths for inoculation, keep the remaining excised sheaths wrapped with a damp paper towel to avoid desiccation.
- Place 20 µL of the fungal spore suspension on the inner surface at the center of the sheath piece, directly above the midrib, as depicted in Figure 4.
- Place the inoculated leaf sheaths horizontally, with the midrib placed at the bottom, in the support inside a glass Petri plate containing a moistened filter paper as depicted in Figure 5.
- Each rack holds up to seven sheaths. Inoculate at least five sheaths per strain to compensate for loss of replicates that may occur during sample preparation or incubation.
- Place the small humidity chambers into a clear storage box lined with moistened germination paper (see Table of Materials).
- Cover the box with the lid. Incubate the box at 23 °C with continuous illumination for the intended time course, which depends on the fungus and on the fungal development stages that will be observed. A summary of the time course for maize sheaths inoculated with C. graminicola is provided in Table 1.
- Check every day for signs/symptoms of disease on the sheaths and keep both germination and filter papers moist. Leaf sheaths can be retained for up to 6 days without obvious plant cell death or degradation in the absence of inoculation.
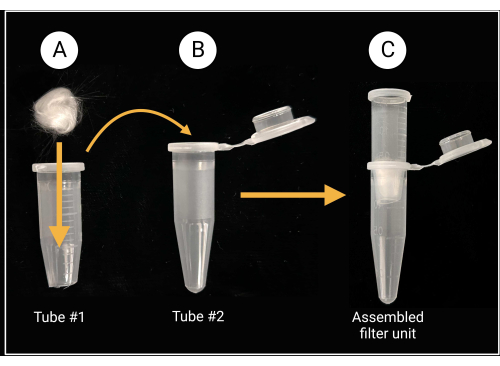
Figure 2: Glass-wool filter unit preparation. (A) A 0.5 cm x 0.5 cm glass-wool ball is placed inside microcentrifuge tube 1 that has its conical bottom removed. (B-C) The filter tube is then placed into microcentrifuge tube 2 to generate an assembled filter unit for spore suspension preparation. Created with BioRender.com. Please click here to view a larger version of this figure.
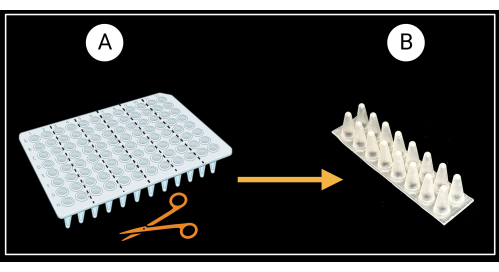
Figure 3: Method of cutting a non-skirted 96-well PCR plate. (A) PCR plate cut into six support racks, 8 x 2 wells. An example of a single sheath support is depicted in (B). Leaf sheaths are laid horizontally on the support. Created with BioRender.com. Please click here to view a larger version of this figure.

Figure 4: Sheath inoculation method. Single drop of inoculum directly applied to the adaxial surface of the sheath section. Please click here to view a larger version of this figure.
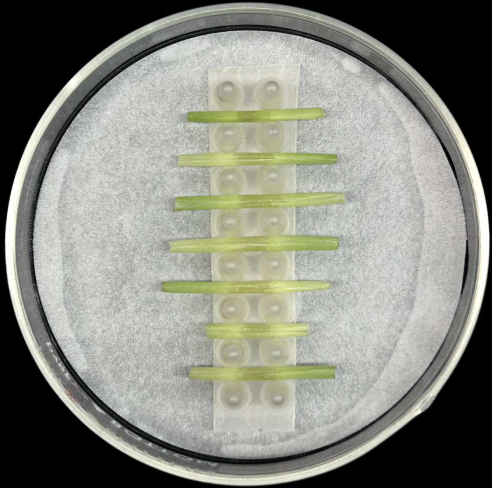
Figure 5: Sheath incubation method. Inoculated leaf sheaths placed horizontally in a support rack inside a glass Petri plate containing moistened filter paper. Please click here to view a larger version of this figure.
3. Live-cell microscopy
- Sample preparation for microscopy
- Rinse sheath pieces gently with sterile DI water to remove any unadhered spores or superficial fungal growth.
- Place the sheath on a clean glass microscope slide (see Table of Materials) with the adaxial (inside) surface of the sheath piece facing down and the abaxial (outside) surface uppermost.
- Use a new single-edge razor blade (see Table of Materials) to trim approximately 1 cm off the sheath ends and remove most of the lamina tissue on either side of the midrib.
- Hold the razor blade at an angle of 90° relative to the sheath to shave tissue from the abaxial midrib and expose the epidermal layer on the adaxial surface above the inoculated spot. Try to maintain a uniform thickness. Once the leaf sheaths are shaved, further trimming is not recommended as this may damage the sample.
- Ensure that the shaved sections are no more than 1 cm x 1 cm in size. Avoid pressing on the center of the sheath during this process. When working with sheaths inoculated with different spore suspensions, disinfect gloves and change the razor blade between samples.
- Keep a clean glass microscope slide ready. Lift the sheath section from one edge and transfer it carefully to a new slide. The inoculated (adaxial) surface of the sheath should be uppermost, closest to the objective lens. Mount sections gently to avoid damage to fungal structures.
- Apply 60 µL of sterile DI water to the section and add a 24 mm x 60 mm glass coverslip (see Table of Materials). Do this slowly to avoid air bubbles.
- When ready for microscopy, seal the coverslip with clear nail polish (see Table of Materials) by placing a small drop on each edge and then connecting the drops together with a nail polish brush to make a seamless seal.
NOTE: This sheath protocol can be used with cytological stains, e.g., 4′,6-diamidino-2-phenylindole (DAPI), neutral red, or trypan blue, or for observation of cell plasmolysis to evaluate plant cell viability. For sheath staining or cell plasmolysis assays, do not seal the coverslip. - For plasmolysis assays, add a hypertonic solution (0.75 M sucrose or 1 M sodium chloride) to one edge of the coverslip and use a folded lint-free tissue paper to draw the solution across the sample to the other edge of the coverslip. For staining, use a similar method to apply the stain solution.
- Widefield microscopy
- Once the leaf sheaths are mounted on microscope slides, inspect them for fungal colonization using a widefield light microscope at 400x magnification.
- To observe fungal structures in detail, use a water immersion objective rather than oil for better image quality of the samples. Spherical aberration due to a refractive-index mismatch may cause image degradation. Water objectives are available for widefield light microscopes as well as confocal microscopes.
- To determine the relative amount of colonization per sheath, count the number of maize cells invaded from each fungal penetration site using a widefield light microscope at 100x magnification. This quantification is an appropriate screening step prior to any statistical analyses comparing strains, treatments, and/or developmental stages.
- Confocal laser scanning microscopy
- To observe fluorescent proteins in maize tissues during development of transgenic fungal strains, adjust the basic image acquisition parameters. Identify the best excitation/emission settings based on the selected fluorescent marker.
NOTE: A 60x water objective is preferable when analyzing fluorescent fusions constructed with secreted proteins. Modern confocal microscopes have highly corrected water immersion objectives to avoid artifacts and shape aberrations. - If live-cell imaging of fluorescent proteins is intended, use untransformed fungal strains as controls for autofluorescence. Use the following image acquisition parameters to capture fungal-host dynamics on an inverted confocal microscope and for mCherry fluorescent protein. Set laser power up to 5%, 450-600 high voltage (HV) depending on the level of expression, 1.375 X gain, 3% offset, an optical zoom factor of 1 for the analysis of up to five maize cells per section, and optical zoom factor of 2-5 for individual hyphae.
NOTE: High-resolution Z-stack confocal images provide three-dimensional data and a better view of real-time interactions between the host and the pathogen. - For quantification of fluorescence intensities corresponding to secreted fusion proteins, use image analysis software from the confocal manufacturer or an image processing program such as ImageJ, which can be freely downloaded online. Consult a manual for details on how to quantify fluorescent signals accordingly.
- To observe fluorescent proteins in maize tissues during development of transgenic fungal strains, adjust the basic image acquisition parameters. Identify the best excitation/emission settings based on the selected fluorescent marker.
Results
The examples below describe representative outcomes following the use of the maize leaf sheath inoculation method. These examples demonstrate the ease, speed, and precision with which observation and comparison of maize-fungus interactions can be accomplished in real-time with this optimized assay. Live-cell imaging also allows the extraction of quantitative information, providing a useful tool for comparative molecular, cytological, and physiological studies. Further details may be found in the original publications cit...
Discussion
The optimized leaf sheath inoculation method described here is modified from an original protocol that was developed for and has been applied to rice leaf sheaths6,8,36. It allows direct, detailed observations of fungal growth and development in living plant cells with either widefield or confocal microscopy. The protocol is suitable for characterization, comparison, and quantification of a variety of microscopic phenomena durin...
Disclosures
The authors declare that they have no competing financial interests and nothing to disclose.
Acknowledgements
The authors thank USDA-NIFA for their financial support (grant numbers 2018-67013-28489 and 2020-70410-32901). Any opinions, findings, conclusions, or recommendations expressed in this manuscript are solely those of the authors and do not necessarily reflect the views of the U.S. Department of Agriculture. We thank Science Without Borders visiting student from Brazil, Mayara de Silva, for the images that appear in Figure 6A and in Figure 7D. We also acknowledge the Department of Plant Pathology at the University of Kentucky for providing access to the Olympus confocal microscopes.
Materials
| Name | Company | Catalog Number | Comments |
| Axiocam monochrome microscope camera | ZEISS | 426560-9010-000 | Compatible with the Axioplan 2 microscope; provides low read noise and high speed for live cell imaging |
| Axioplan 2 epifluorescence microscope | ZEISS | N/A | Allows live viewing and image/video capture of biological samples |
| Benchtop centrifuge 24 X 1.5/2 mL | Thermo Fisher Scientific | 75002431 | Sorvall Legend Micro 17; max speed: 13,300 rpm (17,000 x g) |
| Falcon bacteriological Petri dish with lid | Fisher Scientific | 08-757-105 | Polystyrene material; hydrophobic surface |
| Filter paper | Fisher Scientific | 09-920-115 | Whatman grade 1 for Petri plate moist chambers |
| FV 3000 laser scanning confocal microscope | Olympus | N/A | For visualization of fungal transformants' |
| Germination paper | Anchor Paper Co. | SD7615L | 76# heavy weight for plastic box moist chambers |
| Glass Petri dishes | VWR International | 75845-542 | Type 1 class A, 33 expansion borosilicate glass; complete set (cover + bottom), for Petri plate moist chambers |
| Glass wool | Ohio Valley Specialty Chemical | 3350 | For glass-wool filter units |
| Hemocytometer/Neubauer counting chamber and cover glass | VWR International | 15170-172 | 0.1 mm chamber depth; comes with two 0.4 mm cover glasses |
| Microscope coverslips | Fisher Scientific | 12-553-457 | Borosilicate glass; 100/Pk.; 22 mm length, 22 mm width |
| Maize cultivar Golden Jubilee seeds | West Coast Seeds Ltd., Delta, BC, Canada | CN361 | Matures in 95-105 days; seed type: F1 |
| Microcentrifuge tubes | USA Scientific | 1415-2500 | 1.5 mL capacity |
| Microscope slides | Fisher Scientific | 12-550-123 | Superfrost white tab slide; 76 mm length, 25 mm width |
| Oatmeal Agar (OA) | VWR International | 255210 | Difco Oatmeal Agar, BD; 500 g |
| Nail polish | Revlon | 43671 | Clear nail polish for sealing microscope slides; color 771 Clear |
| Non-skirted 96-well PCR plate | USA Sientific | 1402-9500 | 100 uL plate volume |
| Pestle for microcentrifuge tubes | USA Scientific | 1415-5390 | Conical tip; polypropylene material |
| PlanApo 60X/1,00 WLSM water objective | Olympus | 1-UB933 | Compatible with the Olympus FV 3000 confocal microscope |
| Potato Dextrose Agar (PDA) | VWR International | 90000-758 | Difco Potato Dextrose Media, BD; 500 g |
| Pro-Mix BX | Premium Horticulture Supply Co. | N/A | Premium general-purpose growing medium formulated to provide a balance of water retention and proper drainage |
| SC10 cone-tainers | Greenhouse Megastore | CN-SS-SC-10B | 1.5 inch diameter, 8.25 inch depth, and a volume of 164 mL |
| SC10 cone-tainers tray | Greenhouse Megastore | CN-SS-SCTR98 | 24 inch length x 12 inch width x 6.75 inch height; holds up to 98 of SC10 cone-tainers |
| Single edge razor blade | Thermo Fisher Scientific | 17-989-145 | AccuTec blade; steel material; 38 mm length blade |
| Storage containers/boxes with latch closure | Target | 002-02-0405 | Clear view storage boxes for rmoist chamber; outside dimensions: 23 5/8 inch x 16 3/8 inch x 6 1/2 inch; 32 qt. capacity |
References
- Cheng, Y., Yao, J., Zhang, H., Huang, L., Kang, Z. Cytological and molecular analysis of nonhost resistance in rice to wheat powdery mildew and leaf rust pathogens. Protoplasma. 252 (4), 1167-1179 (2015).
- Hickey, E. L., Coffey, M. D. A fine-structural study of the pea downy mildew fungus Peronospora pisi in its host Pisum sativum. Canadian Journal of Botany. 55 (23), 2845-2858 (1977).
- Wharton, P. S., Julian, A. M., O'Connell, R. J. Ultrastructure of the infection of Sorghum bicolor by Colletotrichum sublineolum. Phytopathology. 91 (2), 149-158 (2001).
- Latunde-Dada, A. O., et al. Infection process and identity of the hemibiotrophic anthracnose fungus (Colletotrichum destructivum O'Gara) from cowpea (Vigna unguiculata (L) Walp.). Mycological Research. 100 (9), 1133-1141 (1996).
- Bourett, T. M., Howard, R. J. In vitro development of penetration structures in the rice blast fungus Magnaporthe grisea. Canadian Journal of Botany. 68 (2), 329-342 (1990).
- Sakamoto, M. On the new method of sheath inoculation of rice plants with blast fungus, Pyricularia oryzae Cav., for the studying of the disease-resistant nature of the plant. Bulletin of the Institute for Agricultural Research, Tōhoku University. 1 (3), 120-129 (1949).
- Buiate, E. A., et al. A comparative genomic analysis of putative pathogenicity genes in the host-specific sibling species Colletotrichum graminicola and Colletotrichum sublineola. BMC genomics. 18 (1), 1-24 (2017).
- Kankanala, P., Czymmek, K., Valent, B. Roles for rice membrane dynamics and plasmodesmata during biotrophic invasion by the blast fungus. The Plant Cell. 19 (2), 706-724 (2007).
- Khang, C. H., et al. Translocation of Magnaporthe oryzae effectors into rice cells and their subsequent cell-to-cell movement. The Plant Cell. 22 (4), 1388-1403 (2010).
- Mosquera, G., Giraldo, M. C., Khang, C. H., Coughlan, S., Valent, B. Interaction transcriptome analysis identifies Magnaporthe oryzae BAS1-4 as biotrophy-associated secreted proteins in rice blast disease. The Plant Cell. 21 (4), 1273-1290 (2009).
- Sakulkoo, W., et al. A single fungal MAP kinase controls plant cell-to-cell invasion by the rice blast fungus. Science. 359 (6382), 1399-1403 (2018).
- Torres, M. F., Cuadros, D. F., Vaillancourt, L. J. Evidence for a diffusible factor that induces susceptibility in the Colletotrichum-maize disease interaction. Molecular Plant Pathology. 15 (1), 80-93 (2014).
- Valent, B., Khang, C. H. Recent advances in rice blast effector research. Current Opinion in Plant Biology. 13 (4), 434-441 (2010).
- Mims, C. W., Vaillancourt, L. J. Ultrastructural characterization of infection and colonization of maize leaves by Colletotrichum graminicola, and by a C. graminicola pathogenicity mutant. Phytopathology. 92 (7), 803-812 (2002).
- Vargas, W. A., et al. Plant defense mechanisms are activated during biotrophic and necrotrophic development of Colletotricum graminicola in maize. Plant Physiology. 158 (3), 1342-1358 (2012).
- Venard, C., Vaillancourt, L. Colonization of fiber cells by Colletotrichum graminicola in wounded maize stalks. Phytopathology. 97 (4), 438-447 (2007).
- Venard, C., Vaillancourt, L. Penetration and colonization of unwounded maize tissues by the maize anthracnose pathogen Colletotrichum graminicola and the related nonpathogen C. sublineolum. Mycologia. 99 (3), 368-377 (2007).
- Berruyer, R., Poussier, S., Kankanala, P., Mosquera, G., Valent, B. Quantitative and qualitative influence of inoculation methods on in planta growth of rice blast fungus. Phytopathology. 96 (4), 346-355 (2006).
- Belisário, R., Robertson, A. E., Vaillancourt, L. J. Maize anthracnose stalk rot in the genomic era. Plant Disease. 106 (9), 2281-2298 (2022).
- Warren, H. L., Nicholson, R. L., Ullstrup, A. J., Sharvelle, E. G. Observations of Colletotrichum graminicola on sweet corn in Indiana. Plant Disease Reporter. 57, 143-144 (1973).
- Ritchie, S. W., Hanway, J. J., Benson, G. O. How a corn plant develops. Special Report, Iowa State University. 48, (1986).
- Tuite, J. . Plant pathological methods: fungi and bacteria. , (1969).
- Wicklow, D. T., Rogers, K. D., Dowd, P. F., Gloer, J. B. Bioactive metabolites from Stenocarpella maydis, a stalk and ear rot pathogen of maize. Fungal Biology. 115 (2), 133-142 (2011).
- Daudi, A., O'Brien, J. A. Detection of hydrogen peroxide by DAB staining in Arabidopsis leaves. Bio-protocol. 2 (18), e263-e263 (2012).
- Benhamou, R. I., Jaber, Q. Z., Herzog, I. M., Roichman, Y., Fridman, M. Fluorescent tracking of the endoplasmic reticulum in live pathogenic fungal cells. ACS Chemical Biology. 13 (12), 3325-3332 (2018).
- Lorang, J. M., et al. fluorescent protein is lighting up fungal biology. Applied and Environmental Microbiology. 67 (5), 1987-1994 (2001).
- Liu, C. Y., Zhu, J., Xie, Z. Visualizing yeast organelles with fluorescent protein markers. JoVE (Journal of Visualized Experiments). 182, e63846 (2022).
- Westermann, B., Neupert, W. Mitochondria-targeted green fluorescent proteins: convenient tools for the study of organelle biogenesis in Saccharomyces cerevisiae. Yeast. 16 (15), 1421-1427 (2000).
- Lee, A. S. The ER chaperone and signaling regulator GRP78/BiP as a monitor of endoplasmic reticulum stress. Methods. 35 (4), 373-381 (2005).
- Krishnakumar, V., et al. A maize database resource that captures tissue-specific and subcellular-localized gene expression, via fluorescent tags and confocal imaging (Maize Cell Genomics Database). Plant and Cell Physiology. 56 (1), e12-e12 (2015).
- Wu, Q., Luo, A., Zadrozny, T., Sylvester, A., Jackson, D. Fluorescent protein marker lines in maize: generation and applications. International Journal of Developmental Biology. 57 (6-7-8), 535-543 (2013).
- O'Connell, R. J., et al. Lifestyle transitions in plant pathogenic Colletotrichum fungi deciphered by genome and transcriptome analyses. Nature Genetics. 44 (9), 1060-1065 (2012).
- Torres, M. F., et al. A Colletotrichum graminicola mutant deficient in the establishment of biotrophy reveals early transcriptional events in the maize anthracnose disease interaction. BMC Genomics. 17 (202), 1-24 (2016).
- Andrie, R. M., Martinez, J. P., Ciuffetti, L. M. Development of ToxA and ToxB promoter-driven fluorescent protein expression vectors for use in filamentous ascomycetes. Mycologia. 97 (5), 1152-1161 (2005).
- Gordon, C. L., et al. Glucoamylase:: green fluorescent protein fusions to monitor protein secretion in Aspergillus niger. Microbiology. 146 (2), 415-426 (2000).
- Koga, H., Dohi, K., Nakayachi, O., Mori, M. A novel inoculation method of Magnaporthe grisea for cytological observation of the infection process using intact leaf sheaths of rice plants. Physiological and Molecular Plant Pathology. 64 (2), 67-72 (2004).
- Xavier, K. V., Pfeiffer, T., Parreira, D. F., Chopra, S., Vaillancourt, L. Aggressiveness of Colletotrichum sublineola strains from Sorghum bicolor and S. halepense to sweet sorghum variety Sugar Drip, and their impact on yield. Plant Disease. 101 (9), 1578-1587 (2017).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved