A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Comparative Study of Basement-Membrane Matrices for Human Stem Cell Maintenance and Intestinal Organoid Generation
In This Article
Summary
Organoids have become valuable tools for disease modeling. The extracellular matrix (ECM) guides cell fate during organoid generation, and using a system that resembles the native tissue can improve model accuracy. This study compares the generation of induced pluripotent stem cells-derived human intestinal organoids in animal-derived ECM and xeno-free hydrogels.
Abstract
Extracellular matrix (ECM) plays a critical role in cell behavior and development. Organoids generated from human induced pluripotent stem cells (hiPSCs) are in the spotlight of many research areas. However, the lack of physiological cues in classical cell culture materials hinders efficient iPSC differentiation. Incorporating commercially available ECM into stem cell culture provides physical and chemical cues beneficial for cell maintenance. Animal-derived commercially available basement membrane products are composed of ECM proteins and growth factors that support cell maintenance. Since the ECM holds tissue-specific properties that can modulate cell fate, xeno-free matrices are used to stream up translation to clinical studies. While commercially available matrices are widely used in hiPSC and organoid work, the equivalency of these matrices has not been evaluated yet. Here, a comparative study of hiPSC maintenance and human intestinal organoids (hIO) generation in four different matrices: Matrigel (Matrix 1-AB), Geltrex (Matrix 2-AB), Cultrex (Matrix 3-AB), and VitroGel (Matrix 4-XF) was conducted. Although the colonies lacked a perfectly round shape, there was minimal spontaneous differentiation, with over 85% of the cells expressing the stem cell marker SSEA-4. Matrix 4-XF led to the formation of 3D round clumps. Also, increasing the concentration of supplement and growth factors in the media used to make the Matrix 4-XF hydrogel solution improved hiPSC expression of SSEA-4 by 1.3-fold. Differentiation of Matrix 2-AB -maintained hiPSC led to fewer spheroid releases during the mid-/hindgut stage compared to the other animal-derived basement membranes. Compared to others, the xeno-free organoid matrix (Matrix 4-O3) leads to larger and more mature hIO, suggesting that the physical properties of xeno-free hydrogels can be harnessed to optimize organoid generation. Altogether, the results suggest that variations in the composition of different matrices affect stages of IO differentiation. This study raises awareness about the differences in commercially available matrices and provides a guide for matrix optimization during iPSC and IO work.
Introduction
The extracellular matrix (ECM) is a dynamic and multifunctional component of tissues that plays a central role in regulating cell behavior and development. As a complex network, it provides structural support, cell adhesive ligands1, and storage of growth factors and cytokines that regulate cell signaling. For example, during wound healing, the ECM serves as a scaffold for migrating cells and as a reservoir of growth factors involved in tissue repair2. Similarly, dysregulation in the ECM can lead to an increase in the severity of various diseases such as fibrosis and cancer3,4. During embryonic development, the ECM guides tissue morphogenesis. For example, in the development of the heart, ECM components play a role in creating the correct architecture and function of the heart tissue5. Over a decade of research has shown that the stiffness of the microenvironment alone6,7 can control stem cell lineage specification. Therefore, it is not surprising that during in vitro cell differentiation, ECM influences stem cell fate by providing signals for differentiation.
Organoids can be generated from induced pluripotent stem cells (iPSCs). Starting with a properly characterized iPSC line is required to generate organoids successfully. However, the lack of physiological cues in classical cell culture materials hinders efficient iPSC differentiation and organoid generation. Moreover, recent research has emphasized the significance of the composition of the extracellular matrix (ECM), interactions between cells and the ECM8, as well as mechanical and geometrical cues9,10,11 in the context of organoid expansion and differentiation12. Advancing organoid technology by improving reproducibility will involve incorporating tissue-specific physical and chemical cues.
Organoids aim to recapitulate the native tissue within a physiologically similar microenvironment. Choosing an ECM system that closely mimics the native tissue ECM is crucial for achieving physiological relevance regarding cell behavior, function, and response to stimuli13. The choice of ECM components can influence the differentiation of stem cells into specific cell types within the organoid. Different ECM proteins and their combinations can provide cues that guide cell fate14. For example, studies have shown that using specific ECM components can promote the differentiation of intestinal stem cells into mature intestinal cell types, resulting in physiologically relevant intestinal organoids15. While organoids are a valuable tool during disease modeling and drug testing, selecting an appropriate ECM system is pivotal to this application. An appropriate ECM system can enhance the accuracy of disease modeling by creating a microenvironment that resembles the affected tissue16. Furthermore, tissue-specific ECM can help generate organoids that better recapitulate disease-associated phenotypes and drug responses17. Optimizing the ECM system used in organoid differentiation is critical for achieving desired differentiation outcomes.
Commercially available basement membrane systems derived from animal ECM sources (e.g., Matrigel, Cultrex) and xeno-free hydrogel (e.g., VitroGel) are widely used in iPSC and organoid research. Companies that commercialize them and researchers that use them have laid out many instructions for their specific products and applications over the years. Many of these instructions served as a guide for the generation of this protocol. Furthermore, the benefits and setbacks associated with their intrinsic properties have been individually noted by many18,19,20,21. However, there is no systematic workflow to guide the selection of optimal systems for iPSC and organoid work. Here, a workflow to systematically evaluate the equivalency of ECM systems from various sources for iPSC and organoid work is provided. This is a comparative study of the maintenance of two different human iPSC lines (hiPSC) and human intestinal organoids (hIO) generation in four different matrices: Matrigel (Matrix 1-AB), Geltrex (Matrix 2-AB), Cultrex (Matrix 3-AB), and VitroGel (Matrix 4-XF). For organoid culture, four versions of the xeno-free matrix VitroGel that were previously optimized for organoid culture were used: ORGANOID 1 (Matrix 4-O1), ORGANOID 2 (Matrix 4-O2), ORGANOID 3 (Matrix 4-O3), ORGANOID 4 (Matrix 4-O4). Also, animal-derived matrices optimized for organoids were used: Matrigel High Concentration (Matrix 1-ABO) and Cultrex Type 2 (Matrix 3-ABO). Commercially available stem cell culture media (mTeSR Plus) and organoid differentiation kit (STEMdiff intestinal organoid kit) were used. This protocol combines the individual instructions from the products' manufacturers with lab experiences to guide the reader toward a successful optimization of ECM for their specific iPSC and organoid work. Altogether, this protocol and representative results emphasize the importance of selecting the optimal microenvironment for stem cell work and organoid differentiation.
Protocol
1. hiPSC maintenance
CAUTION: All work is done in a Biosafety Cabinet (BSC) following standard aseptic techniques. Must follow OSHA safety standards for laboratories, including proper use of personal protective equipment such as lab coats, gloves, and goggles.
- Preparation of matrices aliquots and cell culture media
- For commercially available animal-derived basement membranes (BMs; Matrix 1-AB, Matrix 2-AB, Matrix 3-AB), prepare aliquots of working volumes following the manufacturer's recommendation summarized in Table 1 and store them at -20 °C or -80 °C for long term storage. Avoid the formation of bubbles. If some bubbles are formed, before storing, centrifuge samples at 4 °C, at <200 x g, for ~1-2 min to force the bubble to come to the surface.
NOTE: Making single-use aliquots helps avoid repeated freeze-thaw cycles that disrupt ECM architecture. Because BM concentration varies with lot number, make sure to follow the manufacturer's recommendation to prepare single-use aliquots and make coating solutions. High-concentrated BMs are viscous and difficult to pipette; use cold tips previously stored at -20 °C. - Prepare stem cell culture medium following manufacturer's recommendation. To prepare the specific commercially available complete media used in this protocol, add 100 mL of 5x supplement cocktail to 400 mL of basal medium. Then, mix this media thoroughly and aliquot into 40 mL volumes, stored at 20 °C. For use, thaw each aliquot of complete media, use the aliquoted complete medium immediately, or store at 2 - 8 °C for up to 2 weeks. Do not re-freeze it.
- Prepare aliquots of the stem cell complete medium containing 3x the concentration of the supplement cocktail to prepare the Matrix 4-XE Hydrogel, i.e., dilute 5x supplement to 3x.
- For commercially available animal-derived basement membranes (BMs; Matrix 1-AB, Matrix 2-AB, Matrix 3-AB), prepare aliquots of working volumes following the manufacturer's recommendation summarized in Table 1 and store them at -20 °C or -80 °C for long term storage. Avoid the formation of bubbles. If some bubbles are formed, before storing, centrifuge samples at 4 °C, at <200 x g, for ~1-2 min to force the bubble to come to the surface.
- Coating tissue culture plastic with animal-derived BMs (Matrix 1-3AB)
- Prepare the following before starting: always keep the matrices in ice when thawing and handling to prevent them from gelling. Use cold media to prepare the diluted matrices. Prepare enough replicate wells per condition to assay cells before starting and at every stage of the hiPSC differentiation process and hIO organoid generation.
NOTE: Here, 24-well plates were used for this study. Refer to Table 2 for recommended coating volumes for other plate sizes. - For each type of matrix, prepare 25 mL of cold advance DMEM/F-12 containing 15 mM HEPES in a 50 mL conical tube. Keep these on ice.
- Add the thawed matrices to their respective cold advance DMEM/F-12 and mix well. Keep the media cold during the mixing process. Visually check for homogenous mixing by ensuring there are no clumps.
- Immediately use the diluted matrices solutions to coat each well selected for use (250 µL/ well if using 24 well plates).
- Gently tilt the culture ware to spread the coating solution evenly across the surface. Incubate at room temperature (15 - 25 °C) for at least 1 h before use.
NOTE: If not used immediately, the plate can be stored at 2 - 8 °C for up to 1 week after coating, but it must be sealed with a transparent film to prevent evaporation. When using stored plates, let them reach room temperature (15 - 25 °C) for 30 min before proceeding to the next step. - Gently remove the excess solution using a serological pipette or by aspiration. Ensure that the coated surface is not scratched.
- Add warm complete stem cell medium immediately (50% of total volume needed for specific well, e.g., 250 µL/well if using a 24-well plate).
- Prepare the following before starting: always keep the matrices in ice when thawing and handling to prevent them from gelling. Use cold media to prepare the diluted matrices. Prepare enough replicate wells per condition to assay cells before starting and at every stage of the hiPSC differentiation process and hIO organoid generation.
- Coating tissue culture plastic with Matrix 4-XE hydrogel
- Prepare the following before starting: take the Matrix 4-XE hydrogel out of the refrigerator and let it warm to room temperature (25 °C). Prepare enough replicate wells per condition to assay cells before starting and at every stage of the hiPSC differentiation process and hIO organoid generation.
NOTE: Here, 24-well plates were used for this study. Refer to Table 3 for recommended coating volumes for other plate sizes. - Prepare a complete stem cell culture media containing 3x growth factor concentration compared to the standard formulation for stem cell culture (3x stem cell medium).
- Mix Matrix 4-XE hydrogel and 3x stem cell medium at a 2:1 v/v mixing ratio and gently pipette up and down 5x-10x to mix thoroughly.
- Transfer the hydrogel mixture to a well plate and carefully tilt the culture ware to spread the mixture evenly across the surface. Use 250 µL/well if using a 24-well plate. See Table 3 for recommended volumes.
- Wait 10-15 min at room temperature for soft gel formation. During the hydrogel-formation process, do not disrupt the hydrogel by tilting or shaking the plate.
- Prepare the following before starting: take the Matrix 4-XE hydrogel out of the refrigerator and let it warm to room temperature (25 °C). Prepare enough replicate wells per condition to assay cells before starting and at every stage of the hiPSC differentiation process and hIO organoid generation.
- hiPSC enzyme-free clump passaging and seeding
- Prepare the following before starting: at least 1 h before passaging, coat desired plasticware with matrices. Aliquot sufficient complete stem cell media and warm to room temperature (15 - 25 °C). Avoid multiple heating cycles for the complete media.
NOTE: The steps below describe the passage of an already established and >90% confluent culture of iPSCs on a 6-well plate and seeding them in 24-well plates for differentiation of intestinal organoids. - Rinse cells with 1 mL of D-PBS (without Ca++ and Mg++) and aspirate. Add 1 mL of enzyme-free human pluripotent stem cell selection reagent, carefully swirl to spread evenly, and aspirate within 1 min. Colonies only need to be exposed to a thin film of liquid.
- Incubate at 37 °C until colonies start looking less compacted, which will take ~ 3-8 min.
NOTE: Optimal incubation time may vary depending on the cell line used. The optimal incubation time must be determined when passaging each cell line with the enzyme-free human pluripotent stem cell selection reagent for the first time. - Add 1 mL of complete stem cell media. Detach the colonies by carefully tapping firmly on the side of the plate. Make sure to hold the plate with the other hand.
- With a 1 mL or larger pipette, transfer the cell clumps suspension to a 15 mL conical tube. Evaluate the size of the clumps using a brightfield microscope and make sure the cell clump's size is between 50-200 µm; if they are larger, gently shake the conical tube to break them down.
- Draw an x-y grid at the bottom of each well of a flat-bottom 96-well plate that will be used for clump counting and add 50 µL of D-PBS to each of the wells. It is recommended to average the clumps counted from 3 wells.
- Ensure cell clumps are evenly distributed by gently shaking the tube and then transfer 5 µL of clump suspension into each of the wells of the flat-bottom 96-well plate.
NOTE: It is not recommended to use an automatic cell counter to count clumps. A manual counting is recommended. - Count the total number of clumps in each well that are 50 - 200 µm in diameter. If most cell clumps are > 200 µm in diameter (see Figure 1), repeat steps 1.4.5 - 1.4.7.
- Calculate the volume (in µL) of clump suspension required to seed 6,000 clumps as follows:
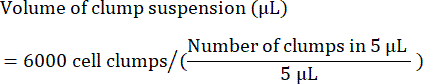
NOTE: Optimal seeding density must be optimized with every cell line. To determine optimal seeding density, it is recommended to perform an initial seeding in a range of clump densities (e.g., 4,000, 5,000, and 6,000 clumps per well) and check 24-48 h after seeding if 85%-90% confluency is reached. - As a quality control, separate a sample of cells for flow cytometry examination of stem cell markers. Refer to Table 4 for a list of common markers.
- Separate the required volume of iPSC clump suspension to seed on the wells coated with different ECM into separate 15 mL tubes.
- Centrifuge the tube containing the iPSC clump solution at 200 x g for 5 min to remove media containing enzyme -free human pluripotent stem cell selection reagent. While waiting for centrifugation, add 50% of the desired volume per well to each well if it was not done already.
- Prepare the following before starting: at least 1 h before passaging, coat desired plasticware with matrices. Aliquot sufficient complete stem cell media and warm to room temperature (15 - 25 °C). Avoid multiple heating cycles for the complete media.
- iPSC culture in plates containing animal-derived matrices
- Gently resuspend iPSCs clumps in the 50% calculated volume of complete stem cell media to get the desired density and plate the cell aggregate mixture onto coated wells containing 50% of calculated complete stem cell media.
NOTE: Adding ROCK-1 inhibitor might be necessary for some cell lines or when clumps are 50 µm or smaller. It is recommended to add <10 µm to avoid early gastrulation. - Carefully add medium with cells on top of the matrices, 250 µL/well if using a 24-well plate. For the recommended volume of cell medium for other size well plates, check Table 2.
- Tilt the plate in several short back-and-forth and side-to-side motions to distribute the cell clumps evenly.
CAUTION: Uneven distribution of aggregates results in increased spontaneous differentiation of human iPSCs. Circular spreading of the clumps' suspension results in clumps agglomerating on the border of the wells and lower density at the center. - Incubate the plate at 37 °C and perform medium changes using complete stem cell media daily or every other day. When performing medium changes, visually assess cultures to monitor growth and determine whether the cells require passaging time or are ready for differentiation. To skip two consecutive days of feeding, add 2x the volume of the medium needed for a single day.
- Gently resuspend iPSCs clumps in the 50% calculated volume of complete stem cell media to get the desired density and plate the cell aggregate mixture onto coated wells containing 50% of calculated complete stem cell media.
- iPSC culture in plates containing Matrix 4-XE
- Gently resuspend iPSCs clumps into the total calculated volume of 3x complete stem cell media to get the desired density.
- Carefully add medium with cells on top of the hydrogel 250 µL/well if using a 24-well plate. For the recommended volume of cell medium for other size well plates, check Table 3.
CAUTION: The hydrogel will swell and occupy a larger volume than freshly made. iPSC colonies will be partially embedded in the hydrogel at the bottom of the plate, so it is recommended to change 50%-80% of the top medium without disturbing the hydrogel. - Move the plate in several quick, short, back-and-forth, and side-to-side motions to distribute the cell clumps evenly.
- Place the plate in a 37 °C incubator with 5% CO2 and 95% humidity. Change the cell medium using complete stem cell media. Perform medium changes daily or every other day; for the latter one, add 2x the volume of the medium.
- Visually inspect the cultures to track their growth as they reach the stage suitable for differentiation.
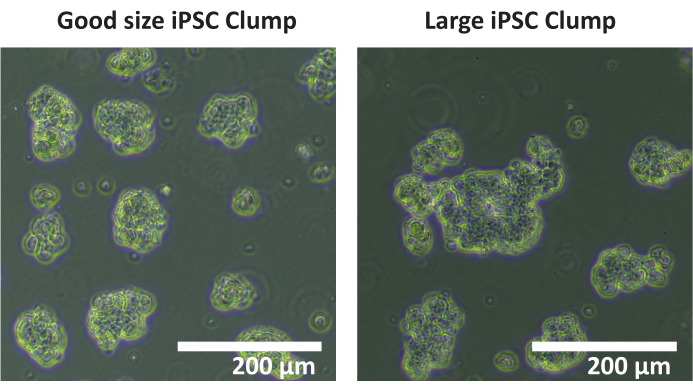
Figure 1: Optimal clump size. Images of clumps of iPSC cell line SCTi003A depicting an example of optimal clump size. Scale bar = 200 µm. Please click here to view a larger version of this figure.
2. hiPSC differentiation and intestinal organoid generation
CAUTION: All work is done in a Biosafety Cabinet (BSC) following standard aseptic techniques. Must follow OSHA safety standards for laboratories, including proper use of personal protective equipment such as lab coats, gloves, and goggles.
- Prepare the following before starting: prepare aliquots of differentiation medium required for each stage. After thawing the aliquots, use them immediately. Do not re-freeze. Follow Table 5 for a general guide of each stage media type and volumes needed for a 24-well plate.
NOTE: Optimal time at each differentiation stage might vary per cell line. Checking the expression of each stage cell marker is recommended to improve differentiation efficacy. Periodical assessment of gene expression on the iPSCS cultures was not included in this research since we leveraged vendor-qualified batches at low passages, but it is recommended as a quality control step for new/not yet qualified iPSC lines during subsequent differentiation steps. - Stage 1: Definitive endoderm (DE)
- To assess readiness to start differentiation, check for the following criteria.
- Using a microscope, evaluate the confluency of the stem cell colonies and the amount of spontaneous differentiation in the culture. Do not rely only on morphological evaluation alone. Optimal confluency should be between 85%-90% (above ~105 cells/cm2), and minimal spontaneous differentiation observed should be <5% differentiation.
- Collect cells from a sacrificial well for flow cytometry characterization of hiPSC markers before proceeding with the next step. A forward and side scatter gating strategy was used for the representative flow cytometry characterization. If cells meet the optimal criteria, start differentiation. Cell passing criteria is >85% expression of ideally 3 markers (Table 4), especially in case of new/not yet qualified iPSC lines in the lab.
NOTE: iPSC cultures grown on different matrices undergo the same overall process with only iPSC growth on Matrix 4-XE, requiring more gentle media changes not to disturb the semi-embedded cells. Significant cell death is expected during the induction of definitive endoderm, as cells are particularly sensitive at this stage. Exercise caution during medium changes, aiming to minimize the duration that cells are exposed outside the 37 °C incubation environment as much as possible.
- Start differentiation of cell colonies as described below.
- Day 0: Warm an aliquot of DE medium to 37 °C. To avoid denaturing of media from warming-cooling cycles, ensure to warm only the volume required for Day 0 (0.7 mL/well).
- Aspirate the media from hiPSCs. Carefully add 0.7 mL of DE Medium per well down the side of the well. Avoid harsh pipetting that could detach or damage the colonies. Incubate the plate at 37 °C with 5% CO2 and 95% humidity for 24 h.
- Day 1: Warm an aliquot of DE medium containing only the volume needed for Day 1 (0.5 mL/well) to 37 °C. Aspirate the DE medium from cells and carefully add 0.5 mL of DE medium per well down the side of the well. Incubate at 37 °C with 5% CO2 and 95% humidity for 24 h.
- Day 3: Cells are ready to be assayed for definitive endoderm formation. Refer to Table 4 for recommended markers. Before proceeding with the next phase, sacrifice a well to perform a flow cytometry characterization of specific markers associated with DE. A forward and side scatter gating strategy was used for the representative flow cytometry characterization. It is recommended that the expression of more than 1 marker be checked, especially in the case of new/not yet qualified iPSC lines in the lab.
- To assess readiness to start differentiation, check for the following criteria.
- Stage 2: Midgut/Hindgut (MH)
- Day 3: Warm only the volume of MH medium needed for day 3 (0.5 mL/well) to 15 - 25 °C. Aspirate DE medium from cells and replace with 0.5 mL of MH Medium. Incubate at 37 °C with 5% CO2 and 95% humidity for 24 h.
- Days 4 - 9: Before daily medium changes, use the microscope at 2x or 4x magnification to assess spheroids formation by checking for visible 3D structures (might happen from day 4); free-floating spheroids (might occur from day 5).
- Once spheroids start detaching, use a 1 mL pipettor to gently transfer the medium from cells to a sterile 15 mL conical tube to assess the number of spheroids detached from cells.
NOTE: Avoid any shear force that might detach 3D structures from the monolayer; once ready, the spheroids will detach on their own. - If less than 50 spheroids are detached, centrifuge at 200 x g for 5 min, remove the old medium, and resuspend spheroids on 0.5 mL/well of medium and transfer to respective well until enough spheroids have matured.
NOTE: The duration of exposure to the midgut/hindgut medium plays a crucial role in determining the regional identity of developing small intestinal organoids, such as duodenum (shorter exposure) or ileum (longer exposure). To achieve more uniform cultures with the same identity, aim to collect spheroids exposed to midgut/hindgut medium simultaneously. - Repeat step 2.3.3 until enough spheroids are detached to embed and initiate human intestinal organoid (hIO) culture.
- Before proceeding with the next phase, separate a sample of spheroids and perform a flow cytometry characterization of specific markers associated with Midgut/Hindgut (MH). A forward and side scatter gating strategy was used for the representative flow cytometry characterization. Refer to Table 4 for a list of commonly used markers. It is recommended that the expression of more than 1 marker be checked, especially in the case of new/not yet qualified iPSC lines in the lab.
- Stage 3: Spheroid embedding
- Prepare the following before starting the procedure.
- Thaw aliquots of Matrix 1-ABO and Matrix 3-ABO on ice. Consider the number of spheroids collected to determine the total amount of matrix needed if 30-40 µL of matrix will be required per dome.
- Prepare 25 mL of cold Advance DMEM/F-12 containing 15 mM HEPES. High-concentrated ECMs are viscous and difficult to pipette. Place a box of sterile 100 µL pipette tips at -20 °C for preparing cold tips that can help the process. Prepare aliquots of Intestinal Organoid Growth Medium (OGM), which requires 4 feeds (0.5 mL/well per feed). See Table 5 for preparation.
- Thaw an aliquot of the OGM supplement on ice. After thawing the aliquots, use them immediately. Do not re-freeze. Store complete OGM at 2-8 °C for up to 2 weeks.
- Prepare OGM containing 3x of the supplement compared to the standard formulation for (3x OGM). Take the Matrix 4-XFO1 thought Matrix 4-XFO4 out of the refrigerator and let them warm to room temperature (25 °C).
- For the animal-derived system, perform the steps described below.
- Place a sterile 24-well tissue culture plate in the incubator to warm to 37 °C while preparing the spheroids and matrices. Let the collected spheroids settle to the bottom of a 15 mL conical tube. Carefully aspirate and discard the supernatant.
NOTE: To make a more accurate comparison of the effect of the matrices, make sure to keep the spheroids collected from the hiPSC differentiated on each matrix in separate tubes. - Add 1 mL of DMEM/F-12 with 15 mM HEPES to the spheroids. Centrifuge at 300 x g for 5 min at room temperature (15 - 25 °C).
- Carefully remove as much of the supernatant as possible. It is recommended to start removing with a 1 mL pipettor and switch to 100 µL and even 10 µL pipettes to remove as much as possible without disturbing the pellet of spheroids.
NOTE: The larger the media volume left, the more diluted the matrices will be. Diluted matrices can lead to gelling issues or softer domes. Softer domes lead to higher chances of collapsing with minimal disturbances. - Using a pipettor with a cold 100 µL pipette tip, add 40 µL/50 spheroids of cold (2 - 8 °C) Matrix 1-ABO or Matrix 3-ABO to their respective tube. Gently distribute spheroids into the matrix by pipetting up and down ~5x. Do not completely empty the pipette tip, which may introduce bubbles.
- Bring the plate from the incubator, and using a cold pipette tip, gently transfer embedded spheroids into the center of the plate following steps in Figure 2. Avoid dispensing Matrix 1-ABO and Matrix 3-ABO too quickly into the culture dish, as this will flatten the dome.
- Carefully transfer the plate into a 37 °C incubator and incubate for 30 min to ensure dome gelation. Be especially careful of any shaking or harsh movement during transport and in the incubator.
- While incubating the dome for gelling, warm at 37 °C a sufficient volume of intestinal OGM (0.5 mL/well) for the number of wells to be used.
NOTE: Warm only the volume needed. Avoid warming-cooling cycles. Make sure the media is warm before adding it to the dome; cold media can lead to the dome collapsing. - After 30 min, carefully add 0.5 mL of intestinal OGM to the side of the well to not disturb the dome. Incubate at 37 °C with 5% CO2 and 95% humidity. Perform a full-medium change every 3 - 4 days by removing the medium and then add fresh medium.
- Place a sterile 24-well tissue culture plate in the incubator to warm to 37 °C while preparing the spheroids and matrices. Let the collected spheroids settle to the bottom of a 15 mL conical tube. Carefully aspirate and discard the supernatant.
- For the Matrix 4-XF organoid systems follow the steps described.
NOTE: Matrix 4-XF organoid system includes four different options (O1-O4); the formulation of each type varies in bio-functional ligands, stiffness, and degradability. Consequently, performing initial experiments to determine the optimal type for the specific application is recommended. This protocol describes using the four options to find an optimal formulation for hiPSC-derived intestinal organoids. Additionally, while there are multiple culture protocols that can be used (e.g., 3D encapsulation, dome encapsulation), this protocol involves using the dome method such that it is possible to compare directly with the common methods used when working with animal-derived basement membrane systems.- Add 1 mL/well of cold advance DMEM/F-12 containing 16 mM HEPES to the spheroids generated on the Matrix 4-XFO4 system. Centrifuge at 300 x g for 5 min at room temperature (15 - 25 °C).
NOTE: To find the optimal formulation for the application, make sure to distribute spheroids into 4 separate tubes before centrifugation. Spheroids generated on animal-derived matrices can also be matured into intestinal organoids using this system. - Using a 1 mL pipettor, remove the supernatant without disturbing the spheroids. Add 50 µL of 3x OGM to the spheroids pellet (~100 spheroids). Add 100 µL of the selected Matrix 4-XFO to the 50 µL spheroid suspension and gently mix 5x-10x times. Keep the Matrix 4-XFO to 3x OGM at a 2:1 v/v mixing ratio to have a final concentration of 1x.
- Add 40 µL of the hydrogel-spheroid mixture to the center of a 24-well tissue culture plate. Carefully transfer the plates into a 37 °C incubator and incubate for 30 min.
NOTE: Matrix 4-XFO does not require 37 °C incubation for gelation; however, it is recommended to keep organoids exposed to similar conditions when compared to Matrix 1-ABO and Matrix 3-ABO systems that require 37 °C for better gelation of the domes. - After 30 min, carefully add 0.5 mL of intestinal OGM to the side of the well so as not to disturb the dome. Incubate at 37 °C with 5% CO2 and 95% humidity. Perform a full-medium change every 3 - 4 days by removing the medium and then adding fresh medium.
- Add 1 mL/well of cold advance DMEM/F-12 containing 16 mM HEPES to the spheroids generated on the Matrix 4-XFO4 system. Centrifuge at 300 x g for 5 min at room temperature (15 - 25 °C).
- Prepare the following before starting the procedure.
- hIO passaging and maturation
- Prepare the same chemicals, solutions, and reagents as done in step 2.4.1.
- Add 1-2 mL of anti-adherence rinsing solution to a 15 mL conical tube (1 per condition) and swirl to coat the tube.
- Remove the anti-adherence solution and rinse the tubes with 5 mL of D-PBS (without Ca++ and Mg++). Cap all coated tubes and keep them at room temperature (15 - 25 °C) until needed.
- Aspirate the medium from the domes. Using a 1 mL pipette, add 1 mL of cold DMEM/F-12 to the dome directly. The goal is to detach the domes from the plate.
- Add an additional 1 mL of cold DMEM/F-12 to the well and pipette up and down to harvest any remaining organoids. Transfer to the coated 15 mL conical tubes.
NOTE: Verify the successful harvest of organoids by visually examining the well under a microscope. If any residual organoids are observed, repeat step 2.5.5. - With a 1 mL pipettor, perform up-and-down pipetting of the suspension to disintegrate organoids until a uniform fragment suspension is achieved with the desired organoid size (e.g., 100 - 500 µm).
NOTE: Utilize a 200 µL pipettor to verify that the organoids conform to the recommended size. Employing the 200 µL pipettor facilitates additional fragmentation as needed, ensuring that the fragments can smoothly pass through a 200 µL pipette tip.
CAUTION: Avoid breaking up fragments into single cells by harsh or prolonged pipetting. - Establish the desired organoid density by either counting fragments or employing the split ratio. Separate an aliquot and perform counting using the same procedure as in steps 1.4.6-1.4.9 for clump counting.
NOTE: Optimal organoid density should be optimized per line; generally, a density of 40 - 80 intestinal organoids per dome is recommended. - Ensure that the tube is placed on ice during the organoid counting process. After approximately 5 min, organoid fragments will have settled to the bottom of the tube due to gravity.
NOTE: The larger the volume of the organoid solution, the longer it will take to settle to the bottom. - Gently eliminate as much supernatant and the cloudy layer formed on top of the organoids. In the early passages when organoids are maturing, this cloudy phase encompasses a matrix and individual cells.
- Add 2 mL of cold DMEM/F-12 by directly pipetting onto the pellet. Centrifuge at 200 x g for 5 min at room temperature (15 - 25 °C).
- For animal-derived system, carefully remove and discard supernatant following the same steps described in steps 2.4.2.4-2.4.2.9.
- For the Matrix 4-XFO system, carefully remove and discard the supernatant following the same steps described in steps 2.4.3.2-2.4.3.4.
NOTE: When working with Matrix 4-XFO system, it is recommended to use a xeno-free hydrogel organoid recovery solution for optimal removal of hydrogel residual. This recovery solution is especially recommended when switching from animal-based to xeno-free hydrogel systems to ensure the elimination of any xenogeneic material.
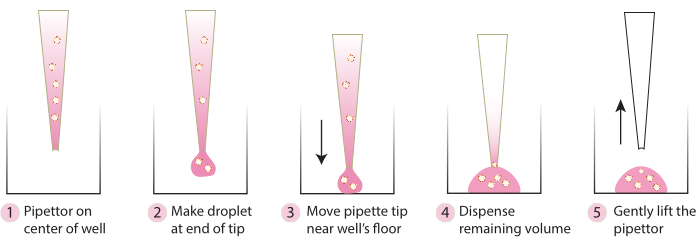
Figure 2. Schematic of technique recommended for dome formation. The schematic describes the step-by-step process recommended for successful dome formation for all systems. Please click here to view a larger version of this figure.
3. IO size characterization
NOTE: The size of the organoids was characterized by brightfield images taken at 4x and 10x. The image processing analysis was automated using MATLAB. The overall steps of the process are described below, and a sample of the code is included in Supplementary File 1.
- Define the directory containing the images and list all the image files in the directory. Initialize the table to store the results. Read the brightfield images in a file.
- Define pixel to µm conversion and set the scale. If the image does not contain a scale bar, request the user the pixel-to-µm conversion factor.
- Convert the image to grayscale. Apply a Gaussian filter to reduce noise in the image. Threshold the filtered image to separate organoids from the background.
- Fill small holes and remove small objects to clean up the binary image. Perform connected component analysis to identify individual organoids and calculate their properties such as area, centroid, major axis length, and minor axis length.
- Calculate organoid size based on the major and minor axis lengths. Display the segmented organoids on the original image and label them with their size. Print the organoid sizes and store results in .cvs file.
NOTE: The first couple of images were analyzed manually to determine the optimal parameters for the Gaussian filter standard deviation and the area to the threshold. The code in Supplementary File 1 provides an example of the basic framework for organoid size analysis from brightfield images; however, further refinement is needed to suit the specific requirements for each image type and quality. The same process can be done using open-source software like FIJI from Image J. - Follow the steps below to use the FIJI software.
- Define pixel to µm conversion and set the scale. If the image does not contain a scale bar, request the user the pixel-to-µm conversion factor by clicking Analyze > Set Scale.
- Convert the image to grayscale by clicking Image > Type > 8bit. Apply a Gaussian filter to reduce noise in the image by clicking Process > Filter > Gaussian blurb > Sigma (radius) used: 2.
- Threshold the filtered image to separate organoids from the background by clicking Image > Threshold > MaxEntropy > Apply.
- Fill small holes and remove small objects to clean up the binary image by clicking Process > Noise > Remove Outliers > 20 pixels.
- Perform connected component analysis to identify individual organoids and calculate their properties such as area, centroid, major axis length, and minor axis length by clicking Analyze > Set measurement > Make sure Area, Perimeter, and Diameter are included > Analyze > Analyze Particles > Show Outlines.
- Statistical analysis
- Assess the data distribution for normality through the Saphiro-Wilk test using JMP (SAS) software. To examine statistical differences between groups, perform a two-way ANOVA, and conduct post-hoc testing using the nonparametric Wilcoxon method in JMP(SAS) software. Significance was established at an alpha level of p ≤ 0.05.
Results
Following this protocol, commercially available basement membranes and a xeno-free hydrogel system were successfully utilized to cultivate hiPSC cells and differentiate them into hIO. The main objective of these experiments was to systematically evaluate the equivalency of matrices from various sources for hiPSC and hIO work. The first section of this protocol focused on the maintenance and characterization of a healthy iPSC culture that yields an efficient intestinal organoid generation. The process of coating the cultu...
Discussion
Selecting the optimal microenvironment for stem cell and organoid work is a pivotal early step when using these platforms for a wide range of applications. Our representative results show that Matrix 4-XFO3, in combination with a higher concentration of growth factors, leads to larger organoids, suggesting that the physical properties of xeno-free hydrogels can be harnessed to optimize organoid generation using these systems. It has been previously shown that the unique characteristics of the extracellular matrix (ECM) a...
Disclosures
Dr. John Huang is founder & CEO at TheWell Bioscience.
Acknowledgements
The authors acknowledge previous training and general recommendations regarding starting hiPSC and organoid work from Drs. Christina Pacak, Silveli Susuki-Hatano, and Russell D'Souza. They thank Dr. Chelsey Simmons for her guidance in using hydrogel systems for in vitro cell culture work. Also, the authors would like to thank Drs. Christine Rodriguez and Thomas Allison from STEMCELL Technologies for their guidance on hiPSC culture. The authors also thank TheWell Bioscience for covering the publication costs.
Materials
| Name | Company | Catalog Number | Comments |
| 24-Well Plate (Culture treated, sterile) | Falcon | 353504 | |
| 37 °C water bath | VWR | ||
| 96-well plate | Fisher Scientific | FB012931 | |
| Advanced DMEM/F12 | Life Technologies | 12634 | |
| Anti-adherence Rinsing Solutio | STEMCELL Technologies | 7010 | |
| Biological safety cabinet (BSC) | Labconco | Logic | |
| Brightfield Microscope | Echo Rebel | REB-01-E2 | |
| BXS0116 | ATCC | ACS-1030 | |
| Centrifuge with temperature control (4 °C capabilities) | ThermoScientific | 75002441 | |
| Conical tubes, 15 mL, sterile | Thermo Fisher Scientific | 339650 | |
| Conical tubes, 50 mL, sterile | Thermo Fisher Scientific | 339652 | |
| Cultrex RGF BME, Type 2 | Bio-techne | 3533-005-02 | |
| Cultrex Stem Cell Qualified RGF BME | Bio-techne | 3434-010-02 | |
| D-PBS (Without Ca++ and Mg++) | Thermo Fisher Scientific | 14190144 | |
| GeltrexLDEV-Free, hESC-Qualified Reduce Growth Factor | Gibco | A14133-02 | |
| GlutaMAX Supplement | Thermo Fischer Scientific | 35050-061 | |
| Guava Muse Cell Analyzer or another flow cytometry equipment (optional) | Luminex | 0500-3115 | |
| HEPES buffer solution | Thermo Fischer Scientific | 15630-056 | |
| Heralcell Vios Cell culture incubator (37 °C, 5% CO2) | Thermo Scientific | 51033775 | |
| JMP Software | SAS Institute | JMP 16 | |
| MATLAB | MathWorks, Inc | R2022b | |
| Matrigel Growth Factor Reduced (GFR) Basement Membrane Matrix LDEV free | Corning | 356231 | |
| Matrigel Matrix High Concentration (HC), Growth Factor Reduced (GFR) LDEV-free | Corning | 354263 | |
| mTeSR Plus Medium | STEMCELL Technologies | 100-0276 | |
| Nunclon Delta surface treated 24-well plate | Thermo Scientific | 144530 | |
| PE Mouse Anti-human CD326 (EpCAM) | BD Pharmingen | 566841 | |
| PE Mouse Anti-human CDX2 | BD Pharmingen | 563428 | |
| PE Mouse Anti-human FOXA2 | BD Pharmingen | 561589 | |
| PerCP-Cy 5.5 Mouse Anti-human SSEA4 | BD Pharmingen | 561565 | |
| ReLeSR | STEMCELL | 5872 | |
| SCTi003-A | STEMCELL Technologies | 200-0510 | |
| Serological pipettes (10 mL) | Fisher Scientific | 13-678-11E | |
| Serological pipettes (5 mL) | Fisher Scientific | 13-678-11D | |
| STEMdiff Intestinal Organoid Growth Medium | STEMCELL Technologies | 5145 | |
| STEMdiff Intestinal Organoid Kit | STEMCELL Technologies | 5140 | |
| Vitrogel Hydrogel Matrix | TheWell Bioscience | VHM01 | |
| VitroGel ORGANOID Discovery Kit | TheWell Bioscience | VHM04-K |
References
- Hynes, R. O. Integrins: Bidirectional, allosteric signaling machines. Cell. 110 (6), 673-687 (2002).
- Frantz, C., Stewart, K. M., Weaver, V. M. The extracellular matrix at a glance. J Cell Sci. 123, 4195-4200 (2010).
- Hinz, B., Gabbiani, G. Fibrosis: Recent advances in myofibroblast biology and new therapeutic perspectives. F1000 Biol Rep. 2, 78 (2010).
- Pickup, M. W., Mouw, J. K., Weaver, V. M. The extracellular matrix modulates the hallmarks of cancer. EMBO Rep. 15 (12), 1243-1253 (2014).
- Rozario, T., DeSimone, D. W. The extracellular matrix in development and morphogenesis: A dynamic view. Dev Biol. 341 (1), 126-140 (2010).
- Even-Ram, S., Artym, V., Yamada, K. M. Matrix control of stem cell fate. Cell. 126 (4), 645-647 (2006).
- Engler, A. J., Sen, S., Sweeney, H. L., Discher, D. E. Matrix elasticity directs stem cell lineage specification. Cell. 126 (4), 677-689 (2006).
- Tran, O. N., et al. Organ-specific extracellular matrix directs trans-differentiation of mesenchymal stem cells and formation of salivary gland-like organoids in vivo. Stem Cell Res Ther. 13 (1), 306 (2022).
- Nikolaev, M., et al. Homeostatic mini-intestines through scaffold-guided organoid morphogenesis. Nature. 585 (7826), 574-578 (2020).
- Gjorevski, N., et al. Designer matrices for intestinal stem cell and organoid culture. Nature. 539 (7630), 560-564 (2016).
- Gjorevski, N., et al. Tissue geometry drives deterministic organoid patterning. Science. 375 (6576), (2022).
- Heo, J. H., Kang, D., Seo, S. J., Jin, Y. Engineering the extracellular matrix for organoid culture. Int J Stem Cells. 15 (1), 60-69 (2022).
- Shamir, E. R., Ewald, A. J. Three-dimensional organotypic culture: experimental models of mammalian biology and disease. Nat Rev Mol Cell Biol. 15 (10), 647-664 (2014).
- Clevers, H. Modeling development and disease with organoids. Cell. 165 (7), 1586-1597 (2016).
- Jung, P., et al. Isolation and in vitro expansion of human colonic stem cells. Nat Med. 17 (10), 1225-1227 (2011).
- Lancaster, M. A., Knoblich, J. A. Organogenesis in a dish: Modeling development and disease using organoid technologies. Science. 345 (6194), 1247125 (2014).
- Huch, M., et al. In vitro expansion of single Lgr5+ liver stem cells induced by Wnt-driven regeneration. Nature. 494 (7436), 247-250 (2013).
- Greenlee, A. R., Kronenwetter-Koepel, T. A., Kaiser, S. J., Liu, K. Comparison of Matrigel and gelatin substrata for feeder-free culture of undifferentiated mouse embryonic stem cells for toxicity testing. Toxicol In Vitro. 19 (3), 389-397 (2005).
- Geltrex LDEV-Free, HESC-Qualified, Reduced Growth Factor Basement Membrane Matrix User Guide (Pub.No. MAN0007336 3.0. Fisher Scientific Available from: https://www.thermofisher.com/document-connect/document-connect.html?url=https://assets.thermofisher.cn/TFS-Assets%2FLSG%2Fmanuals%2FGeltrex_LDEV_Free_hESC_qualified_PI.pdf (2024)
- biotechne R&D Systems. Cultrex Stem Cell Qualified Reduced Growth Factor. biotechne R&D Systems. , (2024).
- VitroGel Organoid Protocol. TheWell Bioscience Available from: https://www.thewellbio.com/video-protocols (2024)
- Spence, J. R., et al. Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature. 470 (7332), 105-110 (2011).
- Henderson, J. K., et al. Preimplantation human embryos and embryonic stem cells show comparable expression of stage-specific embryonic antigens. Stem Cells. 20 (4), 329-337 (2002).
- Haruna, N. F., Huang, J. Investigating the dynamic biophysical properties of a tunable hydrogel for 3D cell culture. J Cytol Tissue Biol. 7, 30 (2020).
- Cherne, M. D., et al. A synthetic hydrogel, VitroGel ORGANOID-3, improves immune cell-epithelial interactions in a tissue chip co-culture model of human gastric organoids and dendritic cells. Front Pharmacol. 12, 707891 (2021).
- Stewart, D. C., et al. Quantitative assessment of intestinal stiffness and associations with fibrosis in human inflammatory bowel disease. PLoS One. 13, e0200377 (2018).
- Hernandez-Gordillo, V., et al. Fully synthetic matrices for in vitro culture of primary human intestinal enteroids and endometrial organoids. Biomaterials. 254, 120125 (2020).
- Broguiere, N., et al. Growth of epithelial organoids in a defined hydrogel. Adv Mater. 30, 1801621 (2018).
- Barthes, J., et al. Cell microenvironment engineering and monitoring for tissue engineering and regenerative medicine: The recent advances. BioMed Res Int. 2014, 921905 (2014).
- Engler, A. J., Sen, S., Sweeney, H. L., Discher, D. E. Matrix elasticity directs stem cell lineage specification. Cell. 126 (4), 677-689 (2006).
- Aisenbrey, E. A., Murphy, W. L. Synthetic alternatives to Matrigel. Nat Rev Mater. 5 (7), 539-551 (2020).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved