A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Stimulated Single Fiber Electromyography (SFEMG) for Assessing Neuromuscular Junction Transmission in Rodent Models
In This Article
Summary
In this study, we demonstrate a refined single fiber electromyography (SFEMG) protocol to allow in vivo measurement of neuromuscular junction (NMJ) transmission in rodent models. A step-by-step approach to the SFEMG technique is described to allow quantification of NMJ transmission variability and failure in rat gastrocnemius muscle.
Abstract
As the final connection between the nervous system and muscle, transmission at the neuromuscular junction (NMJ) is crucial for normal motor function. Single fiber electromyography (SFEMG) is a clinically relevant and sensitive technique that measures single muscle fiber action potential responses during voluntary contractions or nerve stimulations to assess NMJ transmission. The assessment and quantification of NMJ transmission involves two parameters: jitter and blocking. Jitter refers to the variability in timing (latency) between consecutive single-fiber action potentials (SFAPs). Blocking signifies the failure of NMJ transmission to initiate an SFAP response. Although SFEMG is a well-established and sensitive test in clinical settings, its application in preclinical research has been relatively infrequent. This report outlines the steps and criteria employed in performing stimulated SFEMG to quantify jitter and blocking in rodent models. This technique can be used in preclinical and clinical studies to gain insights into NMJ function in the context of health, aging, and disease.
Introduction
Single fiber electromyography (SFEMG) was initially developed by Stålberg and Ekstedt in the 1960s to identify and analyze action potentials from individual muscle fibers, primarily to study muscle fatigue1. SFEMG is the most sensitive clinical technique for the assessment of neuromuscular junction (NMJ) transmission2. SFEMG is conducted by selectively recording single fiber action potentials (SFAPs)3. NMJ transmission can be compromised due to factors like aging4,5 and various neuromuscular disorders such as myasthenia gravis and amyotrophic lateral sclerosis6. Furthermore, conditions such as ischemia, fluctuations in temperature, and the use of neuromuscular blocking agents can result in deficiencies in NMJ transmission, manifested by increased NMJ transmission variability and occurrences of NMJ failure2.
There are two approaches to recording SFEMG: stimulated and voluntary SFEMG. Voluntary SFEMG involves recording SFAPs from two NMJs supplied by the same motor axon using a concentric needle electrode inserted into the muscle being tested during voluntary activation7. Accordingly, voluntary SFEMG requires cooperation from the subject and can only assess low-threshold motor units (those activated during weak contractions)3. Stimulated SFEMG uses a pair of stimulating electrodes to stimulate motor axons while recording SFAPs with an SFEMG needle electrode inserted into the muscle being tested7.
In both voluntary and stimulated SFEMG, jitter and blocking are the two parameters used to assess and quantify NMJ transmission8. Jitter describes the variability in timing (latency) between consecutive SFAPs. During voluntary SFEMG, jitter is quantified by assessing the latency differences between a pair of SFAPs (supplied by the same motor axon) during 50 to 100 consecutive discharges. During stimulated SFEMG, jitter is quantified by assessing the latency differences between the stimulation timing and the onset of the SFAP during 50 to 100 consecutive discharges. Blocking indicates failure of NMJ transmission to trigger an SFAP response, and it can be quantified as the presence or absence of each pair of SFAPs during voluntary SFEMG or for each NMJ during stimulated SFEMG2,7.
While an established and sensitive test in the clinical setting, SFEMG has only been infrequently applied in preclinical research4,5,9,10,11,12,13,14,15,16,17,18. In this report, we outline the approach to performing and analyzing SFEMG recordings in preclinical rodent models. Furthermore, we present representative data that highlights representative findings on SFEMG that indicate impairment of NMJ transmission following administration of a non-depolarizing neuromuscular blocking agent, rocuronium.
Protocol
All protocols were approved and performed in accordance with the regulations set forth by the Institutional Animal Care and Use Committee at the University of Missouri.
1. Animal preparation and anesthesia administration
- Put on appropriate personal protection equipment.
- Prior to the procedure, measure the rat's weight to determine the appropriate dose for weight-based medications and ventilator settings.
- Induce anesthesia with 3%-5% inhaled isoflurane. Once an adequate level of anesthesia is established, position the rat in a prone position and maintain anesthesia with 1%-3% inhaled isoflurane.
- Verify the adequacy of the depth of anesthesia by gently pressing the hindlimb footpad with forceps to observe the absence of a withdrawal response.
- Maintain body temperature at 37 °C.
- Apply veterinarian-approved petroleum-based ointment to the eyes to prevent dryness. Monitor the depth of anesthesia by observing the respiratory rate and assessing for withdrawal responses when applying pressure to the footpad using forceps.
- Shave the hindlimb to be assessed using clippers. After adequate hair removal, using adhesive tape, position the limb to be studied with the ankle fixed at approximately 90° dorsiflexion, the knee in extension, and the hip in abduction.
- Continuously monitor the respirations and heart rate of the rat during the entire experiment.
NOTE: An increase in heart rate is commonly used as an indicator of inadequate anesthetic depth. - Administer isoflurane to euthanize the rat, with a dose of 5% or greater, until breathing has ceased for at least 3 min. Confirm the euthanasia by decapitation.
2. Electrode placement and setup
NOTE: NMJ transmission of the sciatic nerve and gastrocnemius muscle are assessed using the electromyography (EMG) system. Refer to the Table of Materials.
- Insert a pair of insulated 28 G monopolar needles for stimulating the sciatic nerve with the cathode into the region of the proximal hind limb, while the anode is more proximally within the subcutaneous tissue overlaying the sacrum.
- Ensure the stimulating electrodes are not placed in immediate proximity to the sciatic nerve or excessively deep to avoid direct harm to the sciatic nerve or other adjacent structures.
- Place a disposable ground electrode on the contralateral hindlimb or tail.
- Carefully place a 27-gauge, 25 mm needle electrode specifically designed for single fiber EMG, featuring a recording surface made of platinum-iridium material into the right gastrocnemius muscle. Ensure that the SFEMG electrodes are autoclaved prior to each use to maintain sterility.
- Insert the SFEMG needle electrode in parallel with the gastrocnemius muscle fibers to capture single-fiber action potentials (SFAPs).
- Avoid muscle damage while inserting and maneuvering the SFEMG needle within the muscle.
3. Stimulated single-fiber electromyography (SFEMG) procedure
- Apply constant current stimulation to the right sciatic nerve at 10 Hz frequency using an intensity range of 0.3-10 mA and a pulse duration of 0.1 ms.
- Configure the filter settings within a low frequency filter of 1 kHz and high frequency filter of 10 kHz. Adjust the Gain from 200 µV to 1000 µV per division to facilitate the visualization of potentials. Set the sweep speed at 500 µs per division.
- Adjust the stimulus intensity to trigger and isolate SFAPs for recording and subsequent analysis.
- To identify and analyze a response as an SFAP, ensure that the specific criteria mentioned below are met: Ensure the rise time from the baseline peak to the negative phase is less than 500 µs, the minimum amplitude (baseline peak to negative) is at least 200 µV, and the response consistently exhibit an all-or-none behavior (stable size and shape between responses).
NOTE: It is essential that the recurring responses exhibit consistent upward phases without any notches or inflection points. Note that the last criterion is crucial for distinguishing a quality signal from the summation of multiple signals.
- To identify and analyze a response as an SFAP, ensure that the specific criteria mentioned below are met: Ensure the rise time from the baseline peak to the negative phase is less than 500 µs, the minimum amplitude (baseline peak to negative) is at least 200 µV, and the response consistently exhibit an all-or-none behavior (stable size and shape between responses).
- Calculate Jitter, or variability of SFAP latency (time between stimulation and rising phase of negative peak of SFAP) between consecutive discharges, following at least 50 stimulations (50-100 stimulations), and assess and quantify blocking.
NOTE: Blocking can be assessed as the presence or absence at each synapse or as the percentage of stimulations that fail to trigger single fiber action potential generation. Jitter is calculated using the following equation7 (Jitter is typically automatically calculated by clinic electromyography systems):
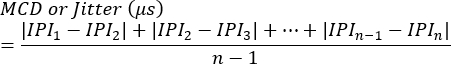
MCD = Mean value of Consecutive Difference
IPI = Interpotential Interval - Repeat the process for additional SFAP responses. On average, assess 10 synapses from each animal to calculate jitter and subsequently determine the average values per animal.
- Exclude SFAPs with jitter of less than 4 µs from the analyses to prevent the inclusion of potentials that might have been evoked by direct muscle stimulation11.
- When recording potentials displaying intermittent blocking, increase the stimulus intensity to verify that SFAP failure is not attributable to submaximal stimulation.
Results
To demonstrate increased jitter and blocking in the context of NMJ transmission failure, stimulated SFEMG was performed with and without intravenous administration of rocuronium. Rocuronium is an intermediate-acting, non-depolarizing neuromuscular blocking agent widely used in clinical settings to induce muscle paralysis during surgeries or medical procedures. It operates by competitively binding to nicotinic acetylcholine receptors at the NMJ19. Prior to the administration of rocuronium, the adul...
Discussion
SFEMG is commonly used for diagnostic testing in patients with suspected autoimmune, acquired, and genetic forms of NMJ disease. SFEMG is considered the most sensitive test for the diagnosis of the NMJ disorder, myasthenia gravis20,21. Repetitive nerve stimulation (RNS) is another method that is more commonly used in clinical diagnostic testing and involves stimulating a peripheral nerve with a train of stimuli and quantifying the summated compound muscle action ...
Disclosures
W. David Arnold received research funding from NMD Pharma and Avidity Biosciences and is consulting for NMD Pharma, Avidity Biosciences, Dyne Therapeutics, Novartis, Design Therapeutics, and Catalyst Pharmaceuticals.
Acknowledgements
The authors would like to thank Dr. Martin Brandhøj Skov from NMD Pharma for his valuable advice on rocuronium dosing and Arash Karimi from the Biomedical Engineering Department of Stony Brook University for his assistance in calculations. This study was supported in part by funding from NIH to WDA (R01AG067758 and R01AG078129).
Materials
| Name | Company | Catalog Number | Comments |
| 27 G Reusable Single Fiber Needle Electrode | Technomed | 202860-000 | singlefiber recording electrode |
| 2 mL Glass Syringe | Kent Scientific Corporation | SOMNO-2ML | |
| Detachable Cable | Technomed | 202845-0000 | to connect the recorder electrode to the electrodiagnostic machine |
| Disposable 2" x 2" disc electrode with leads | Cadwell | 302290-000 | ground electrode |
| disposable monopolar needles 28 G | Technomed | 202270-000 | cathode and anode stimulating electrodes |
| EMG needle cable (Amp/stim switch box) | Cadwell | 190266-200 | to connect monopolar electrodes to electrodiagnostic stimulator |
| Helping Hands alligator clip with iron base | Radio Shack | 64-079 | Maintaining recording electrode placement |
| Isoflurane (250 mL bottle) | Piramal Healthcare | NA | |
| monoject curved tip irrigating syringe | Covidien | 81412012 | utilized for application of electrode gel |
| PhysioSuite Physiological Monitoring System with RightTemp Homeothermic Warming | Kent Scientific Corporation | PS-RT | Includes infrared warming pad, rectal probe, and pad temperature probe |
| Pro trimmer Pet Grooming Kit | Oster | 078577-010-003 | clippers for hair removal |
| Rat Endotracheal Tubes (16 G) | Kent Scientific Corporation | ||
| Rocoronium Bromide | Sigma | PHR2397-500MG | neuromuscular blocker agent |
| Sierra Summit EMG system | Cadwell Industries, Inc., Kennewick, WA | NA | portable electrodiagnostic system |
| SomnoSuite Low-Flow Digital Anesthesia System | Kent Scientific Corporation | SOMNO | Includes anti-spill, anti-vapor bottle top adapter; Y adapter tubing; charcoal scavenging filter |
| Veterinarian petroleum-based ophthalmic ointment | Puralube | 26870 | applied during anesthesia to avoid corneal injury |
References
- Stalberg, E. Propagation velocity in human muscle fibers in situ. Acta Physiol Scand Suppl. 287, 1-112 (1966).
- Stålberg, E., Trontelj, J. V. The study of normal and abnormal neuromuscular transmission with single fibre electromyography. J Neurosci Methods. 74 (2), 145-154 (1997).
- Sanders, D. B., et al. Guidelines for single fiber EMG. Clin Neurophysiol. 130 (8), 1417-1439 (2019).
- Chugh, D., et al. Neuromuscular junction transmission failure is a late phenotype in aging mice. Neurobiol Aging. 86, 182-190 (2020).
- Padilla, C. J., et al. Profiling age-related muscle weakness and wasting: Neuromuscular junction transmission as a driver of age-related physical decline. GeroScience. 43 (3), 1265-1281 (2021).
- Selvan, V. A. Single-fiber EMG: A review. Ann Indian Acad Neurol. 14 (1), 64-67 (2011).
- Sanders, D. B., Kouyoumdjian, J. A., Stålberg, E. V. Single fiber electromyography and measuring jitter with concentric needle electrodes. Muscle Nerve. 66 (2), 118-130 (2022).
- Juel, V. C. Single fiber electromyography. Handb Clin Neurol. 160, 303-310 (2019).
- Chung, T., et al. Evidence for dying-back axonal degeneration in age-associated skeletal muscle decline. Muscle Nerve. 55 (6), 894-901 (2017).
- Iyer, C. C., et al. Follistatin-induced muscle hypertrophy in aged mice improves neuromuscular junction innervation and function. Neurobiol Aging. 104, 32-41 (2021).
- Meekins, G. D., Carter, G. T., Emery, M. J., Weiss, M. D. Axonal degeneration in the trembler-j mouse demonstrated by stimulated single-fiber electromyography. Muscle Nerve. 36 (1), 81-86 (2007).
- Gooch, C. L., Mosier, D. R. Stimulated single fiber electromyography in the mouse: Techniques and normative data. Muscle Nerve. 24 (7), 941-945 (2001).
- Chung, T., Tian, Y., Walston, J., Hoke, A. Increased single-fiber jitter level is associated with reduction in motor function with aging. Am J Phys Med Rehabil. 97 (8), 551-556 (2018).
- Sokolow, S., et al. Impaired neuromuscular transmission and skeletal muscle fiber necrosis in mice lacking na/ca exchanger 3. J Clin Investig. 113 (2), 265-273 (2004).
- Añor, S., et al. Evaluation of jitter by stimulated single-fiber electromyography in normal dogs. J Vet Intern Med. 17 (4), 545-550 (2003).
- Mizrachi, T., et al. NMO-IgG and AQP4 peptide can induce aggravation of eamg and immune-mediated muscle weakness. J Immunol Res. 2018, 5389282 (2018).
- Lin, T. S., Cheng, T. J. Stimulated single-fiber electromyography in the rat. Muscle Nerve. 21 (4), 482-489 (1998).
- Finley, D. B., Wang, X., Graff, J. E., Herr, D. W. Single fiber electromyographic jitter and detection of acute changes in neuromuscular function in young and adult rats. J Pharmacol Toxicol Methods. 59 (2), 108-119 (2009).
- Khan, Z. H., Hajipour, A., Zebardast, J., Alomairi, S. R. Muscle relaxants in anesthesia practice: A narrative review. Arch Anesthesiol Crit Care. 4 (4), 547-552 (2018).
- Padua, L., Caliandro, P., Stålberg, E. A novel approach to the measurement of motor conduction velocity using a single fibre emg electrode. Clin Neurophysiol. 118 (9), 1985-1990 (2007).
- Khoo, A., Hay Mar, H., Borghi, M. V., Catania, S. Electrophysiologic evaluation of myasthenia gravis and its mimics: Real-world experience with single-fiber electromyography. Hosp Pract. 50 (5), 373-378 (2022).
- Nannan, G., et al. A role of lamin a/c in preventing neuromuscular junction decline in mice. J Neurosci. 40 (38), 7203 (2020).
- Alley, D. E., et al. Grip strength cutpoints for the identification of clinically relevant weakness. J Gerontol A Biol Sci Med Sci. 69 (5), 559-566 (2014).
- Juel, V. C. Clinical Neurophysiology of Neuromuscular Junction Disease. Handbook of Clinical Neurology. 161, 291-303 (2019).
- Rich, M. M. The control of neuromuscular transmission in health and disease. Neuroscientist. 12 (2), 134-142 (2006).
- Juel, V. C. Evaluation of neuromuscular junction disorders in the electromyography laboratory. Neurol Clin. 30 (2), 621-639 (2012).
- Arnold, W. D., Clark, B. C. Neuromuscular junction transmission failure in aging and sarcopenia: The nexus of the neurological and muscular systems. Ageing Res Rev. 89, 101966 (2023).
- Kokubun, N. Reference values for concentric needle single fiber EMG. Rinsho Shinkeigaku. 52 (11), 1246-1248 (2012).
- Testelmans, D., et al. Rocuronium exacerbates mechanical ventilation-induced diaphragm dysfunction in rats. Crit Care Med. 34 (12), 3018-3023 (2006).
- Suzuki, K., et al. Intravenous infusion of rocuronium bromide prolongs emergence from propofol anesthesia in rats. PLoS One. 16 (2), e0246858 (2021).
- Stålberg, E., et al. Reference values for jitter recorded by concentric needle electrodes in healthy controls: A multicenter study. Muscle Nerve. 53 (3), 351-362 (2016).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved