Method Article
Procedures for In Vitro Cultivation of Treponema pallidum, the Syphilis Spirochete
In This Article
Summary
This protocol describes in vitro cultivation of the syphilis pathogen Treponema pallidum subsp. pallidum in co-culture with mammalian cells. The method is scalable; it can be used to produce large quantities of T. pallidum and to generate clonal cultures.
Abstract
For over a century, Treponema pallidum subsp. pallidum, the spiral-shaped bacterium that causes syphilis, could only be propagated by inoculation and harvest of the organisms from rabbit testes. In 2018, we described a method to continuously cultivate T. pallidumin vitro. This system utilizes co-culture with rabbit epithelial cells (Sf1Ep cells) in a serum-containing tissue culture medium called TpCM-2. The T. pallidum doubling time in culture is similar to that estimated to occur during natural infection (about 33-45 h). The organism can be cultured continuously with a standard passage time of 1 week in a low oxygen (1.5%) environment at 34 °C. This article contains the protocols for culturing T. pallidum, methods for growing and maintaining the required tissue culture cells, and the technique for generating isogenic strains by limiting dilution. The ability to grow T. pallidum in vitro provides new experimental avenues to study and understand this enigmatic organism.
Introduction
Treponema pallidum is a species of spiral-shaped bacteria (called spirochetes) that causes syphilis and related infections in humans and other primates. Syphilis is a serious disease with long-term effects on infected individuals, and it is estimated that over 8 million new cases of syphilis occur worldwide each year1. T. pallidum has been subdivided into three subspecies based on the diseases they cause in humans as well as minor genetic differences: subspecies pallidum (which causes the sexually transmitted disease syphilis), subspecies pertenue (yaws), and subspecies endemicum (causing bejel or endemic syphilis)2,3. T. pallidum subsp. pertenue also causes infections in baboons, chimpanzees, and other primates. A closely related organism called Treponema paraluiscuniculi (also called Treponema paraluisleporidarum) causes an infection in rabbits and hares4,5. All of these bacteria are very closely related, with greater than 98% DNA sequence identity at the genome level6,7,8. They each have a single, small circular chromosome about 1.14 million base pairs (Mb) in size. The members of this T. pallidum group are found only in association with their mammalian hosts; as such, they are obligate pathogens that are dependent on their host species for survival and growth9,10.
Attempts to culture T. pallidum in vitro began shortly after its identification by Schaudinn and Hoffman in 190511,12. However, these efforts failed to lead to consistent, reproducible growth of the organism. As a result, T. pallidum research studies required propagation of the organism through the experimental infection of laboratory animals, most commonly the rabbit13,14. In 1981, Fieldsteel et al.15 introduced a tissue culture system that promoted the multiplication of T. pallidum strains for a period of up to 2 weeks. This system involved co-culture of T. pallidum with Sf1Ep cottontail rabbit epithelial cells in a modified tissue culture medium (T. pallidum Culture Medium 1, TpCM-1) based on Eagle's Minimum Essential Medium (MEM) and 20% fetal bovine serum (FBS). Other culture conditions required were incubation at 34 °C in an atmosphere containing 1.5% O2 and 5% CO29,16. In this system, T. pallidum attaches to the Sf1Ep cells and multiplies when in close association with the mammalian cell surface. Despite many subculture attempts and other modifications, the Fieldsteel et al. system failed to promote continuous in vitro growth.
In 2018, our laboratory reported that the use of a modified medium called TpCM-2 (in which Eagle's MEM was replaced with a more complex tissue culture medium, CMRL 1066) provided T. pallidum the required nutrients to permit consistent long-term culture17. To date, this modification has led to a consistent, continuous culture of at least 5 strains of T. pallidum subsp. pallidum (Nichols, SS14, Mexico A, UW231B, and UW249B) and one strain of T. pallidum subsp. endemicum (Bosnia A)18,19. As an example, the Nichols strain has now been cultured continuously in vitro for over 6 years. Thus far, attempts to culture yaws isolates (T. pallidum subsp. pertenue) or T. paraluiscuniculiin vitro have been unsuccessful18. The TpCM-2 system still requires the presence of Sf1Ep cells, low oxygen concentrations, and incubation at 34 °C, making the system more complex than most bacterial culture techniques. However, this modified T. pallidum culture system has been useful for further defining the bacterium's growth requirements18, determining minimal inhibitory concentrations (MICs) of antimicrobial compounds and peptides20,21,22,23,24,25, propagating new strains from patient tissue aspirates26, isolating clonal populations of the organism27, characterizing the tprK antigenic variation system27,28, examining gene expression29, and performing mutational analysis30,31,32.
Here, we describe the current methods for cultivating T. pallidum in vitro. We hope this information will help facilitate the more widespread application of this in vitro culture technique to the improved diagnosis, treatment, and prevention of syphilis and related treponemal infections.
Protocol
NOTE: All steps require the use of an aseptic technique and sterile materials and reagents. Utilization of a tissue culture laminar flow hood is recommended to reduce both a) the exposure of personnel to infectious material and b) the possibility of microbial contamination of cultures.
1. Establishing Sf1Ep cell stocks
NOTE: Sf1Ep cottontail rabbit epithelial cells can be purchased as frozen stocks from the American Type Culture Collection (see Table of Materials). The slow-growing nature and low metabolic rate of Sf1Ep cells appear to be key to their ability to support the long-term survival and growth of T. pallidum33; therefore, substitution with other mammalian cell cultures is not recommended. Sf1Ep cells are not an immortalized cell line and can be maintained for only 25-30 passages in culture. Therefore, it is important to maintain a frozen stock of low-passage Sf1Ep cells for future use. Immortalized Sf1Ep lines occasionally arise during the long-term culture of Sf1Ep cells. (unpublished observations). These lines often grow faster and are easier to handle; however, sometimes, they lose the ability to support T. pallidum growth. Immortalized Sf1Ep lines can be used and then replaced when the spirochetes begin to grow slowly.
- Prepare a Sf1Ep cell medium and prewarm it in a 37 °C, 5% CO2 incubator.
NOTE: Sf1Ep cell medium consists of Eagle's MEM supplemented with 10% FBS, 1x MEM non-essential amino acids, 2 mM L-glutamine, and 1 mM sodium pyruvate. (see Table of Materials). The medium should be filter sterilized and can be stored at 4 °C for up to 2 months. Antibiotics (such as penicillin and streptomycin) should not be used in the Sf1Ep cell medium because carryover of even trace amounts of the antibiotics will interfere with the growth of T. pallidum. - Thaw the frozen Sf1Ep stock quickly at 37 °C. Wipe down the outside of the vial with 70% ethanol.
- Add 1 mL of Sf1Ep cell medium to the cryovial and mix gently. Add the medium/cell stock mixture to a sterile 15 mL conical centrifuge tube containing 5 mL of Sf1Ep cell medium and gently mix.
- Pellet the cells by centrifugation at 100 x g for 7 min. Remove and discard the supernatant, taking care not to disturb the cell pellet.
- Gently resuspend the thawed Sf1Ep cells in 15 mL of fresh Sf1Ep cell medium and transfer them to a T75 tissue culture flask.
NOTE: Recovery of the newly thawed Sf1Ep cells is improved by centrifugation to remove the DMSO used to freeze the cells. However, steps 1.4-1.7 can be omitted and the thawed cells from step 3 can be directly seeded into a T75 tissue culture flask containing 14 mL of Sf1Ep cell medium. After overnight incubation, replace one-half of the medium with fresh Sf1Ep medium to dilute the residual DMSO. - Incubate the Sf1Ep cultures in a standard humidified tissue culture incubator at 37 °C, 5% CO2. Loosen the caps of non-vented tissue culture flasks to maintain proper medium pH.
2. Passaging Sf1Ep cells
NOTE: Sf1Ep cell culture growth is monitored with an inverted microscope using phase contrast optics. Typically, the cells take about one week to reach near confluency. When the cells reach ~90% confluency, they can be passed, used for T. pallidum cultivation, or preparation of frozen stocks. The culture life can be extended to two weeks by replacement of one-half of the culture medium after one week of culture.
- Aspirate and discard the Sf1Ep growth medium from the flask. Rinse the cell layer with 5 mL of sterile PBS at room temperature (RT), and aspirate and discard the PBS rinse.
- Add 2.5 mL of trypsin-EDTA to the flask and seal the cap. Rock the flask back and forth to cover the cell layer with trypsin-EDTA and incubate the flask at 37 °C for 5 min.
- Tap the flask gently to dislodge the cells. Observe under an inverted microscope to confirm the dispersal of the Sf1Ep cells.
- Add 5 mL of Sf1Ep growth medium and rock the flask to mix with the trypsin-EDTA and stop the action of the trypsin. Remove the suspended Sf1Ep cells to a sterile conical tube.
- Quantitate the cells using a hemocytometer or automated cell counter.
- To maintain working cell stocks, transfer an aliquot (0.5-1.0 mL or ~8 x 105 cells) of the medium/trypsin-EDTA/cell mixture to a new T75 tissue culture flask containing 15 mL of fresh Sf1Ep medium.
- For T. pallidum culture, dilute the cells in Sf1Ep medium to 0.25-0.5 x 105 cells/mL and seed into appropriate culture vessels (Table 1).
- To freeze Sf1Ep cells, spin in a tabletop centrifuge at 100 x g for 7 min. Carefully remove the supernatant without disturbing the cell pellet. Resuspend the cell pellet in Sf1Ep medium supplemented with 10% tissue culture grade DMSO.
- Distribute 1 mL of the cell suspension to each cryovial and freeze overnight at -70 °C to -80 °C in an insulated container (such as a Styrofoam test tube holder) to enhance retention of viability before transferring the vials to a liquid nitrogen cryogenic vessel.
3. Cultivating T. pallidum
CAUTION: All T. pallidum subspecies and strains are pathogenic to humans and are classified as Biosafety Level 2 (BSL-2) Pathogens34. Appropriate measures are necessary to protect personnel; these include the use of gloves and other personal protective equipment (PPE) as well as disinfection of surfaces, materials, and liquids potentially exposed to T. pallidum. T. pallidum is readily inactivated by exposure to 70% ethanol or commercially available disinfectants. Consistent use of laminar flow hoods for handling specimens containing T. pallidum is recommended.
NOTE: T. pallidum is a microaerophilic organism that can be killed by a few hours of exposure to atmospheric levels of oxygen9,16,35. Therefore, it is recommended that the handling of T. pallidum in air be limited to less than an hour if possible. Also, the TpCM-2 medium should be pre-equilibrated in 1.5% O2, 5% CO2, balance N2, and vigorous stirring (e.g., use of a vortex) should be limited. Because T. pallidum culture is typically carried out in the absence of antibiotics, extra care is needed to avoid contamination with bacteria or fungi. The Sf1Ep-TpCM-2 procedure for culturing T. pallidum is summarized in Figure 1 and involves multiple steps, including the seeding of culture vessels with Sf1Ep cells, preparation of the TpCM-2 medium, and inoculation of the cultures with T. pallidum. Heat-inactivated fetal bovine serum (FBS) is a critical medium component, and its efficacy varies among different suppliers and lots19. Prescreening of FBS lots for efficacy is necessary.
- Select the appropriate culture size.
NOTE: T. pallidum culture is scalable from large formats (such as 75-cm2 flasks yielding ~1 x 109 T. pallidum per culture) to 96-well plates (suitable for cloning experiments)17,18,19,27.- When adjusting the culture size, take into account the number of Sf1Ep cells and T. pallidum inoculated per culture, as well as the amount of medium needed, as shown in Table 1. Use flasks with vented caps as they allow free circulation of gases with decreased loss of volume due to evaporation.
- Use the 6-well plate format for initial cultures because it is convenient to include triplicate replicates and extra wells in case microbial contamination occurs.
- Seed Sf1Ep cells 1-2 days prior to the experiment.
- Prepare a suspension of Sf1Ep cells by trypsinization of stock cultures, as described in step 2.7.
- Determine the concentration of Sf1Ep cells in the suspension using a hemacytometer or automated cell counter.
- Add the appropriate number of Sf1Ep cells and Sf1Ep medium (Table 1) to each culture. Incubate the cultures at 37 °C in a standard tissue culture incubator with 5% CO2 until use.
- Prepare TpCM-2 1 day prior to the experiment.
NOTE: TpCM-2 can be prepared and stored at -20 °C for several months. The medium should be thawed and equilibrated in the low-oxygen incubator the day prior to the experiment.- Obtain sterile solutions for the components of TpCM-2 (Table 2) commercially or prepare them from dry reagents and filter-sterilize them. Store the solutions at 4 °C for up to 2 months. Adjust the pH of the MOPS buffer to 7.5 prior to filter sterilization; otherwise, the components or the final TpCM-2 need not be pH-adjusted.
NOTE: The use of sterile, tissue culture-grade distilled water for the preparation of the medium components is recommended. - Combine the reagents listed in Table 2 in a sterile container, adding dithiothreitol (DTT) as a dry powder last (to minimize its oxidation). Scale up (or down) the quantities of each component to prepare the needed amount of TpCM-2. Gently mix and filter-sterilize the medium using a 0.22 µm filter unit.
- Loosen the lid of the flask containing the TpCM-2. Pre-equilibrate the medium by placing it in an anaerobic (Brewer) jar, evacuating and refilling with a 95% N2, 5% CO2 gas mixture three times, and then filling with 1.5% O2, 5% CO2, balance N2 gas mixture after the final evacuation. An example of a system for carrying out this gas exchange process is depicted in Figure 2.
- Quickly transfer the medium to a tri-gas incubator set up to provide a 1.5% O2, 5% CO2, and balance N2 atmosphere at 34 °C. Alternatively, the anaerobic jar containing the medium can be sealed following the gas exchange described in 3.3.3 and transferred to a standard incubator.
- Obtain sterile solutions for the components of TpCM-2 (Table 2) commercially or prepare them from dry reagents and filter-sterilize them. Store the solutions at 4 °C for up to 2 months. Adjust the pH of the MOPS buffer to 7.5 prior to filter sterilization; otherwise, the components or the final TpCM-2 need not be pH-adjusted.
- On the morning of the experiment, check the Sf1Ep cultures using an inverted microscope. Ensure the cells are attached and 5%-10% confluent. Aseptically remove the medium.
- Rinse the wells briefly using a small volume (0.2 mL to 2 mL, depending on vessel size) of the pre-equilibrated TpCM-2, remove the rinse, and add the appropriate amount of TpCM-2 (Table 1). Equilibrate the plates in a 1.5% O2, 5% CO2, and balance N2 atmosphere at 34 °C for 3-4 h as described previously.
- Transfer the plates to a laminar flow hood and inoculate with the appropriate number (Table 1) of T. pallidum from frozen stocks or trypsinized preparations from freshly harvested cultures (as described below). Freshly prepared or frozen stock collected aseptically from infected rabbits13 may also be used. Re-equilibrate the plates as described in 3.3.3 and incubate the cultures in a 1.5% O2, 5% CO2, and balance N2 atmosphere at 34 °C.
4. Harvesting and passaging T. pallidum cultures
NOTE: Because the majority of T. pallidum in culture is attached to the surface of the Sf1Ep cells, it is necessary to dissociate the treponemes from the mammalian cells in order to retrieve them and obtain an accurate count of the organisms. Such 'harvesting' and passage to fresh cultures is typically done on day 7 of culture. The procedure described here is for 6-well plates; the amount of trypsin-EDTA solution used is adjusted up or down depending on the size of the culture format17,19,27.
- At the time of harvest, remove the cultures from the incubator. Examine the Sf1Ep cell layer in each well using an inverted phase contrast microscope and record the cell density (e.g., 80% confluent) and appearance. Also, note the color of the TpCM-2; the resazurin indicator will often turn from pink to yellow as a result of lower pH.
- Pipette the medium from each well into a sterile 15 mL conical tube, using separate pipets for each well to prevent cross-contamination. Rinse each well with 0.35 mL of prewarmed Trypsin-EDTA solution, and add the rinse to the medium.
- Add another 0.35 mL of Trypsin-EDTA solution to each well, and incubate the plate for 5 min in a standard 37 °C incubator; a low O2 atmosphere is not required for this brief time period.
- Check for the rounding up and detachment of the Sf1Ep cells, which also correlates with the dissociation of T. pallidum from the mammalian cells. Monitor this process using the inverted microscope, and provide additional time or Trypsin-EDTA solution as needed. The dissociation process is facilitated by gently rapping the side of the immobilized plate with a plastic test tube rack or similar object.
- Pipette the reserved medium and rinse into the well to retrieve the dissociated T. pallidum and cells. Record the total volume retrieved for calculation of the yield per culture.
- In most experiments, transfer a set volume of the harvested T. pallidum to culture plates with fresh Sf1Ep cells and TpCM-2. In such cases, transfer about 1/20th of the culture volume (such as 200 µl for a 4 mL, 6-well plate culture); adjust this volume up or down depending on whether the T. pallidum strain is fast or slow growing. Remove the Sf1Ep cells in the inoculum by centrifugation at 100 x g for 5 min, but this step is not necessary for routine transfers.
- Immediately after the plates for an experiment are inoculated, exchange the atmosphere in the plates using the evacuation and refilling process (step 3.3.3). Incubate the plates at 34 °C within the Brewer jar or transfer them to a tri-gas incubator.
- Enumerate T. pallidum by darkfield microscopy using a Helber counting chamber or similar device, following the manufacturer's instructions.
NOTE: The Helber chamber is a calibrated glass slide and cover slip that greatly improves the accuracy and reproducibility of the bacterial counts; the chamber is easily disinfected, cleaned, and dried using 70% ethanol and paper tissues and can be reused indefinitely. Ideally, the darkfield microscope should have a 40x objective and 15x eyepieces. Perform duplicate counts for each culture, and record the number of motile and nonmotile T. pallidum and any morphologic changes be recorded. Quantitative PCR (qPCR) may also be used in cases where precise quantitation and determination of motility are not required17,24. - In experiments where treatment with trypsin may not be desirable (such as those examining T. pallidum protein content), use an EDTA Dissociation Medium to dissociate the T. pallidum and the cell monolayer17,19.
NOTE: Dissociation Medium consists of FBS that has been dialyzed against phosphate-buffered saline (PBS) or Earle's Basic Salt Solution (EBSS) without calcium chloride and magnesium chloride to remove divalent cations in a simplified T. pallidum cultivation medium (Table 2). This procedure may take a longer time period (up to 30 min) or repeated treatment for full dissociation.
5. Freezing and storing T. pallidum cultures
NOTE: T. pallidum can be stored indefinitely at or below -70 °C, with viability upon thawing typically being 50%-90%.
- Freeze T. pallidum cultures at harvest with the addition of 10% (v/v) glycerol. Disperse the glycerol throughout the preparation through gentle pipetting or inversion. Then, distribute the preparation in 1-2 mL aliquots into screw-capped freezer vials and immediately place the vials in a -80 °C freezer or a liquid N2 freezer.
- To start a T. pallidum culture from frozen stock, first, prepare appropriate culture vessel(s) containing Sf1Ep cells and TpCM-2 as described in section 3. Thaw the vial containing the T. pallidum frozen stock rapidly; careful use of a 37 °C water bath or heating block is helpful in this regard.
- Then, transfer the thawed preparation to the culture vessel(s). Ensure that the ratio of frozen stock volume to Tp-CM2 medium is 1:5 or greater to ensure sufficient dilution of glycerol to promote the survival and growth of T. pallidum.
- Incubate the culture under microaerobic conditions for 7 days and transfer to fresh cultures as described in section 4.
6. Generating isogenic clones of T. pallidum
NOTE: This procedure is described in detail in a prior study27.
- In a typical experiment, prepare and pre-equilibrate two 96-well plates with 1000 Sf1Ep cells and 200 µL of TpCM-2 per well, as described in section 3.
NOTE: A multichannel 200 µL pipettor and compatible sterile disposable reagent reservoirs greatly simplify the Sf1Ep cell inoculation, medium exchange, and inoculation steps. - Quantitate the concentration of T. pallidum in a freshly harvested preparation using a darkfield microscope and a Helber chamber (step 4.8). Dilute the T. pallidum suspension in TpCM-2 to produce two preparations with concentrations of 10 treponemes/mL and 40 treponemes/mL; 10 mL of each preparation is more than sufficient to inoculate one 96-well plate with each dilution. As a control, prepare 1 mL of another dilution containing 2 x 103 T. pallidum/mL.
- Using either a standard single-channel pipette or a multichannel pipette, inoculate 50 µL per well of the 10 T. pallidum/mL preparation into one of the prepared 96 well plates, omitting two control wells. Repeat this process with the 40 T. pallidum/mL preparation and the other plate. In the two control wells in each plate, inoculate the 2 x 103 T. pallidum/mL dilution. This process will produce plates with (on average) 0.5 or 2 T. pallidum per well, along with positive control wells containing 100 T. pallidum.
NOTE: The plating efficiency of T. pallidum is low under these conditions, so even wells seeded with ~2 organisms are likely to yield clonal populations. - Equilibrate the plates with the low O2 gas mixture (step 3.3.3) and incubate them in a Brewer jar or tri-gas incubator at 34 °C.
- At 7 days, remove 100 µL of medium from each culture well and replace it with 100 µL of fresh, equilibrated TpCM-2. Check the viability and growth of T. pallidum in the control wells by darkfield microscopy and enumeration to ensure that the culture conditions support T. pallidum multiplication.
NOTE: Be sure to use a new pipet tip for each well to prevent cross-contamination of clonal cultures. - At 14 days, transfer 50 µL of the culture supernatant from each well to fresh plates prepared as in step 6.1.
- Repeat alternating feeding and passage as needed on days 21 and 28.
- Monitor the presence of T. pallidum in each well using darkfield microscopy or qPCR26.
NOTE: With the slow growth rate of T. pallidum and necessary loss of organisms during feeding and transfer, the wells seeded with 0.5 or 2 T. pallidum are typically not positive by either method until day 28 or thereafter. - Once positive wells are identified, trypsinize and transfer these wells to 24-well plates for further expansion. Determine clonality by the predominance of a single tprK sequence and the presence of single sequences at sites that are heterogeneous in the parent strain27.
Results
Using the described conditions, T. pallidum typically retains>90% motility and multiplies logarithmically with a doubling time of 33 h to 45 h for approximately 7 days before entering the stationary phase (Figure 3). Over the course of 1 week, the spirochetes undergo approximately 4-5 doublings (Figure 4). ). In addition, different strains of T. pallidum may grow at different rates. Strains of the SS14 group of T. pallidum tend to have slower doubling times than those of the Nichols group17.
Feeding of cultures may extend culture time by several days but the Sf1Ep cell layer often fails after a week of culture. Furthermore, the treponemes reach an upper limit of organisms of about 5 x 107/mL. Cultures transferred at 7 day intervals typically continue logarithmic multiplication with little or no lag phase. Organisms in the stationary phase often become difficult to pass.
Most T. pallidum are attached to the Sf1Ep cells. However, enough T. pallidum remains in the supernatant that medium samples can be removed periodically to check for viability and multiplication. If careful quantitation is required, the volume of the removed medium should be measured, the number of T. pallidum quantitated, and the total number of organisms removed added to the final counts at harvest.
In previous studies, the cloning efficiency (number of cultures positive per organism inoculated) were 12.5% for 2 T. pallidum inoculated per well and 6.7% for 0.5 T. pallidum inoculated per well27. Thus, it is likely that any positive well represents the outgrowth of a single organism at either of these inocula. However, the clonality of the resulting populations must be verified by examining the culture for homogeneity at sites that are heterogeneous in the parent culture. The most definitive way to demonstrate that the culture is isogenic is through whole genome sequencing27.
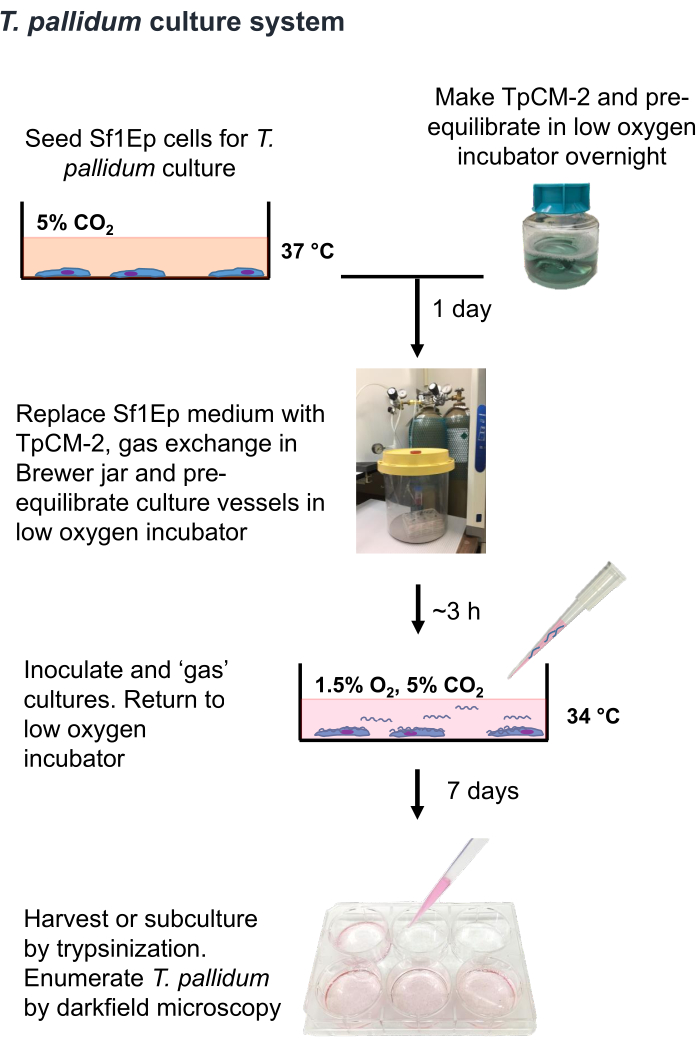
Figure 1: Flow chart of the T. pallidumin vitro cultivation procedure. This figure has been reprinted with permission from Edmondson and Norris (2021)19. Please click here to view a larger version of this figure.
Figure 2: Diagram of the system to equilibrate T. pallidum culture reagents to a low-oxygen environment. A Brewer jar vent is connected via a T-joint to a vacuum source (such as a house vacuum) and to gas cylinders containing custom gas mixtures (5% CO2, balance nitrogen and 1.5% O2, 5% CO2, balance nitrogen). An in-line vacuum gauge measures the vacuum drawn in the jar. The vacuum is drawn in the jar to approximately -58 kPa. The evacuated jar is then slowly re-filled with the gas mixtures. The Brewer jar is refilled three times with 5% CO2, balance nitrogen before a final evacuation, and refilled with 95% N2, 5% CO2, 1.5% O2. Cultures or media are then removed from the jar and quickly transferred to the low-oxygen incubator. Alternately, the tubing between the Brewer jar and the first T-joint can be clamped tightly, the tubing disconnected from the T-joint, and the entire Brewer jar can be transferred to a 34 °C incubator. This figure has been reprinted with permission from Edmondson and Norris (2021)19. Please click here to view a larger version of this figure.
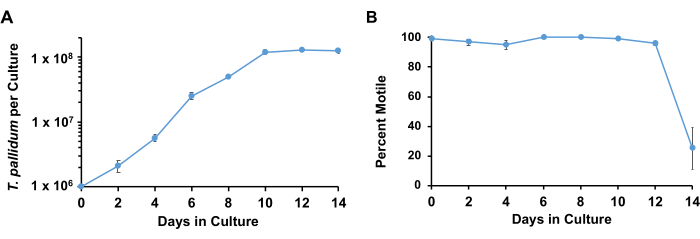
Figure 3: Growth curves of T. pallidum cultured with Sf1Ep cells with TpCM-2 medium. Parallel triplicate cultures were seeded with T. pallidum. Replicates were harvested at each time point; the results represent the mean + SEM for these cultures. (A) The changes in T. pallidum per culture and (B) percent motility are shown. This figure has been adapted with permission from Edmondson et al.18. Please click here to view a larger version of this figure.
Figure 4: Example of passage of in vitro culture of T. pallidum, Nichols strain. Parallel triplicate cultures were seeded with T. pallidum and passed weekly using a 1:20 dilution. The sawtooth plot shows the numbers of T. pallidum per culture and the number transferred to new cultures at each time point. Results represent the mean ± SEM for three biological replicates. Please click here to view a larger version of this figure.
Table 1: Medium volume and seeding ratios for culture vessels. Please click here to download this Table.
Table 2: Media for T. pallidum cultivation. All media should be filter sterilized after preparation. Sf1Ep medium may be stored at 4 °C for up to two months. TpCM-2 is typically made one day prior to use. The dissociation medium should be aliquoted and frozen. Please click here to download this Table.
Discussion
The Sf1Ep-TpCM-2 system is the first available procedure that promotes the continuous in vitro culture of T. pallidum. The system is complex due to the extreme growth requirements of this organism: 1) complex nutritional needs because of limited biosynthetic capabilities; 2) a poorly understood requirement for small quantities of oxygen, despite high sensitivity to reactive oxygen species9,10,16,36; and 3) the current need for the presence of Sf1Ep cells. While it is tempting to 'cut corners' on the procedure, it is recommended that the steps be followed carefully until a successful long-term culture is achieved prior to trying modifications. As additional information about the metabolic requirements of T. pallidum accumulates, it may be possible to develop axenic conditions that do not require the presence of Sf1Ep cells. However, the in vitro growth rate will likely remain slow (with a minimum doubling time of 33 h to 46 h, depending on the strain)17,18, given that T. pallidum multiplies at an estimated doubling time of 30 h to 33 h even during mammalian infection37,38. As with any bacterial culture, it is recommended that low passage stocks be maintained and that experiments be carried out with T. pallidum cultures that are less than 10 passages from these stocks to avoid 'genetic drift' and associated phenotypic changes due to mutations.
Sf1Ep cells apparently provide essential nutrients or enzymatic activities to the treponemes. However, they also consume nutrients (such as glucose and oxygen) and may produce toxic conditions such as low pH9,16,39. Therefore, there is a balancing act between having sufficient Sf1Ep cells to support T. pallidum multiplication and preventing mammalian cell overgrowth and toxicity. High passage numbers of Sf1Ep cells tend to be faster growing and at times, lose the ability to support T. pallidum multiplication. As such, Sf1Ep passage number should be monitored, and cell stocks should be replaced with low-passage frozen preparations periodically. The presence of Sf1Ep cells also complicates the study of T. pallidum properties such as DNA, RNA, and protein content, and enzyme activities. Removal of the rabbit cells is possible to some extent using repeated low-speed centrifugation (100 x g for 5 min) or more effectively using Percoll or Hypaque gradients40,41. However, the gradient centrifugation methods are generally only effective with high numbers of T. pallidum. Alternative methods for propagating T. pallidum are limited to infection of laboratory animals such as rabbits13,14. This approach has ethical considerations and has become increasingly expensive; however, the rabbit model is very useful for studying T. pallidum pathogenesis and host immune responses. In addition, there are likely some differences in gene expression, growth or behavior of T. pallidum during in vitro culture and rabbit infection27.
At the time of this report, the Sf1Ep-TpCM-2 system has been established in at least 6 research groups in the United States and Europe and has resulted in 16 publications with topics ranging from T. pallidum basic biology and genetics to antimicrobial susceptibility. The value of in vitro culture in studying this enigmatic pathogen will likely increase with expanded usage and future improvements.
Disclosures
The authors have no conflicts of interest to disclose.
Acknowledgements
This work was supported by grant R01 AI141958 from the United States National Institutes of Health/NIAID. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Materials
| Name | Company | Catalog Number | Comments |
| 0.5 M EDTA, pH 8.0 | Sigma | E8008 | |
| 10x Earle’s Balanced Salts, w/o Mg2+, Ca2+ | Gibco | 14155063 | |
| 15 and 50 mL conical sterile disposable centrifuge tubes | N/A | N/A | |
| 2 mL cryogenic vials | Corning | 430659 | |
| 6-well cell culture plates for T. pallidum cultivation | Falcon | 353046 | The plates must have low evaporation lids. |
| 70% ethanol | N/A | N/A | |
| 75 cm2 tissue culture flasks with vented caps | Corning | 43061U | |
| 93.5% nitrogen, 5% CO2, and 1.5% oxygen for pre-equilibrating medium and cultures | N/A | N/A | |
| 95% nitrogen and 5% CO2 for pre-equilibrating medium and cultures | N/A | N/A | |
| 96-well low evaporation clear, flat-bottom tissue culture-treated microplates | Corning Falcon | 353072 | |
| Adjustable multi-channel pipette with 200 ul capacity | N/A | N/A | Optional, but very helpful for cloning |
| Cell culture grade water | Sigma | W3500 | |
| CMRL 1066 without L-Glutamine or Phenol Red | US Biological | C5900-03A | |
| CO2 for tri-gas and tissue culture incubators | N/A | N/A | |
| Cryogenic liquid nitrogen cell culture storage tank | N/A | N/A | |
| D-glucose | Sigma-Aldrich | G6152 | |
| Disposable filter units, 0.2 µm , > 100 mL capacity | N/A | N/A | |
| Disposable pipets: 25 mL, 10 mL, 5 mL, aspirating | N/A | N/A | |
| DL-Dithiothreitol | Sigma-Aldrich | D9779 | |
| D-Mannitol | Sigma-Aldrich | M1902 | |
| DMSO (sterile cell culture grade ) | Sigma-Aldrich | D2650 | |
| Eagle’s MEM | Sigma-Aldrich | M4655 | |
| Fetal bovine serum, heat inactivated | Sigma-Aldrich | F4135 | We highly recommend this product. Must pre-screen for T. pallidum culture compatibility if using a different brand or catalog number. |
| Freezer with capability of maintaining -70 °C or -80 °C | N/A | N/A | For storage of T. pallidum; liquid nitrogen storage may be used instead |
| Freezing medium (Sf1Ep medium + 10% [v/v] DMSO) | N/A | N/A | |
| Gas cylinders with appropriate fittings | N/A | N/A | |
| GasPak 150 vented anaerobic jar (Brewer Jar) | Fisher Scientific | 11-816 | |
| Glycerol | N/A | N/A | |
| Helber counting chambers with Thoma rulings | Hawksley Medical and Laboratory Equipment | For quantitating T. pallidum | |
| Hemocytometer | N/A | N/A | For Sf1Ep cell quantitation |
| Incubator tank switch | NuAire | NU-1550 TankGuard Automatic CO2 Incubator Tank Switch | Optional, but very helpful in maintaining appropriate O2 conditions. |
| Inverted microscope with phase contrast optics | N/A | N/A | For viewing Sf1Ep cell cultures |
| L-Glutamine | Sigma-Aldrich | G7513 | |
| L-Histidine | Sigma-Aldrich | H6034 | |
| MEM Non-Essential Amino Acids | Gibco | 11140-050 | |
| Microscope with darkfield condensor | N/A | N/A | The microscope should have a 40x objective and 15x eyepieces. |
| MOPS | Sigma-Aldrich | M3183 | |
| Multi-channel adapter for aspirator | Integra | 155520 | Optional, but useful for cloning |
| NaHCO3 (7.5%) | Sigma-Aldrich | S8761 | |
| Nitrogen for tri-gas incubator | N/A | N/A | |
| Resazurin | Sigma-Aldrich | R7017 | |
| Sf1Ep (NBL-11) cells | American Type Culture Collection | CCL-68 | |
| Sodium pyruvate | Sigma-Aldrich | S8636 | |
| Sterile PBS (without calcium chloride and magnesium chloride) | Sigma-Aldrich | D8537 | |
| Sterile reagent reservoirs, 50 or 100 mL size | N/A | N/A | |
| T. pallidum sample, frozen or fresh | from a rabbit infection or in vitro culture | ||
| Tissue culture incubator maintained at 37 °C, 5% CO2 | N/A | N/A | |
| Tri-gas tissue culture incubator maintained at 34 °C, 5% CO2, 1.5% O2 | Thermofisher | Heracell™ VIOS 160i Tri-Gas CO2 Incubator | Optional; anaerobic jars may be used instead (see Ref. 17) |
| Trypsin-EDTA solution | Sigma-Aldrich | T4049 | |
| Vacuum source (e.g. house vacuum), vacuum tubing, vacuum gauge, and connectors | N/A | N/A | |
| Water, suitable for cell culture, filter-sterilized, purified | Sigma-Aldrich | W3500 | Recommended for medium preparation; decreases culture variability |
References
- Implementing the global health sector strategies on HIV, viral hepatitis and sexually transmitted infections, 2022-2030: Report on progress and gaps. World Health Organization. , Available from: https://www.who.int/publications/i/item/9789240094925 2022-2030 (2024).
- Antal, G. M., Lukehart, S. A., Meheus, A. Z. The endemic treponematoses. Microbes Infect. 4 (1), 83-94 (2002).
- Norris, S. J., Paster, B. J., Smibert, R. M. Bergey's Manual of Systematic Bacteriology. 4, Springer. New York, NY. (2010).
- Lumeij, J. T., Mikalová, L., Šmajs, D. Is there a difference between hare syphilis and rabbit syphilis? Cross infection experiments between rabbits and hares. Vet Microbiol. 164 (1-2), 190-194 (2013).
- Knauf, S., et al. High prevalence and genetic diversity of Treponema paraluisleporidarum isolates in European lagomorphs. Microbiol Spectr. 12 (1), e0177423(2024).
- Šmajs, D., et al. Complete genome sequence of Treponema paraluiscuniculi, strain Cuniculi A: the loss of infectivity to humans is associated with genome decay. PLoS One. 6 (5), e20415(2011).
- Šmajs, D., Norris, S. J., Weinstock, G. M. Genetic diversity in Treponema pallidum: implications for pathogenesis, evolution and molecular diagnostics of syphilis and yaws. Infect Genet Evol. 12 (2), 191-202 (2012).
- Šmajs, D., Strouhal, M., Knauf, S. Genetics of human and animal uncultivable treponemal pathogens. Infect Genet Evol. 61, 92-107 (2018).
- Norris, S. J., Cox, D. L., Weinstock, G. M. Biology of Treponema pallidum: correlation of functional activities with genome sequence data. J Mol Microbiol Biotechnol. 3 (1), 37-62 (2001).
- Radolf, J. D., et al. Treponema pallidum, the syphilis spirochete: making a living as a stealth pathogen. Nat Rev Microbiol. 14 (12), 744-759 (2016).
- Schaudinn, F. R., Hoffman, E. Vorläufiger bericht über das Vorkommen für Spirochaeten in syphilitischen Krankheitsprodukten und bei Papillomen. Arb Gesundh Amt Berlin. 22, 528-534 (1905).
- Schaudinn, F., Hoffmann, E. Über Spirochaetenbefunde im Lymphdrüsensaft Syphilitischer. Deut Med Wochenschr. 31 (18), 711-714 (1905).
- Turner, T. B., Hollander, D. H. Biology of the treponematoses. World Health Organization. , (1957).
- Lukehart, S. A., Marra, C. M. Isolation and laboratory maintenance of Treponema pallidum. Curr Protoc Microbiol. , Chapter 12, Unit 12A.1 (2007).
- Fieldsteel, A. H., Cox, D. L., Moeckli, R. A. Cultivation of virulent Treponema pallidum in tissue culture. Infect Immun. 32, 908-915 (1981).
- Cox, D. L. Culture of Treponema pallidum. Meth Enzymol. 236, 390-405 (1994).
- Edmondson, D. G., Hu, B., Norris, S. J. Long-term in vitro culture of the syphilis spirochete Treponema pallidum subsp. pallidum. mBio. 9 (3), e01153-e01218 (2018).
- Edmondson, D. G., DeLay, B. D., Kowis, L. E., Norris, S. J. Parameters affecting continuous in vitro culture of Treponema pallidum strains. mBio. 12 (1), e03536-e03620 (2021).
- Edmondson, D. G., Norris, S. J. In vitro cultivation of the syphilis spirochete Treponema pallidum. Curr Protoc. 1 (2), e44(2021).
- Edmondson, D. G., Wormser, G. P., Norris, S. J. In vitro susceptibility of Treponema pallidum subsp. pallidum to doxycycline. Antimicrob Agents Chemother. 64 (10), e00979-e01020 (2020).
- Leimer, N., et al. A selective antibiotic for Lyme disease. Cell. 184 (21), 5405-5418 (2021).
- Haynes, A. M., et al. Efficacy of linezolid on Treponema pallidum, the syphilis agent: A preclinical study. EBioMedicine. 65, 103281(2021).
- Houston, S., et al. Identification and functional characterization of peptides with antimicrobial activity From the syphilis spirochete, Treponema pallidum. Front Microbiol. 13, 888525(2022).
- Tantalo, L. C., et al. Antimicrobial susceptibility of Treponema pallidum subspecies pallidum: an in-vitro study. Lancet Microbe. 4 (12), e994-e1004 (2023).
- Hayes, K. A., Dressler, J. M., Norris, S. J., Edmondson, D. G., Jutras, B. L. A large screen identifies beta-lactam antibiotics which can be repurposed to target the syphilis agent. NPJ Antimicrob Resist. 1 (1), 4(2023).
- Tantalo, L. C., Molini, B. J., Bose, M., Klausner, J. D., Giacani, L. In vitro isolation of Treponema pallidum subsp. pallidum from fresh and frozen needle aspirates of primary experimental syphilis lesions. Sex Transm Dis. 50 (3), 180-183 (2023).
- Edmondson, D. G., De Lay, B. D., Hanson, B. M., Kowis, L. E., Norris, S. J. Clonal isolates of Treponema pallidum subsp. pallidum Nichols provide evidence for the occurrence of microevolution during experimental rabbit infection and in vitro culture. PLoS One. 18 (3), e0281187(2023).
- Lin, M. J., et al. Longitudinal TprK profiling of in vivo and in vitro-propagated Treponema pallidum subsp. pallidum reveals accumulation of antigenic variants in absence of immune pressure. PLoS Negl Trop Dis. 15 (9), e0009753(2021).
- De Lay, B. D., Cameron, T. A., De Lay, N. R., Norris, S. J., Edmondson, D. G. Comparison of transcriptional profiles of Treponema pallidum during experimental infection of rabbits and in vitro culture: Highly similar, yet different. PLoS Pathog. 17 (9), e1009949(2021).
- Romeis, E., et al. Genetic engineering of Treponema pallidum subsp. pallidum, the syphilis spirochete. PLoS Pathog. 17 (7), e1009612(2021).
- Phan, A., Romeis, E., Tantalo, L., Giacani, L. In vitro transformation and selection of Treponema pallidum subsp. pallidum. Curr Protoc. 2 (8), e507(2022).
- Romeis, E., et al. Treponema pallidum subsp. pallidum with an artificially impaired TprK antigenic variation system is attenuated in the rabbit model of syphilis. bioRxiv. , 524629(2023).
- Fieldsteel, A. H., Becker, F. A., Stout, J. G. Prolonged survival of virulent Treponema pallidum (Nichols strain) in cell-free and tissue culture systems. Infect Immun. 18, 173-182 (1977).
- U.S. Department of Health and Human Services. Biosafety in Microbiological and Biomedical Laboratories (BMBL) 6th Edition. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Institutes of Health. , (2020).
- Norris, S. J., Miller, J. N., Sykes, J. A., Fitzgerald, T. J. Influence of oxygen tension, sulfhydryl compounds, and serum on the motility and virulence of Treponema pallidum (Nichols strain) in a cell- free system. Infect Immun. 22 (3), 689-697 (1978).
- Cox, C. D., Barber, M. K. Oxygen uptake by Treponema pallidum. Infect Immun. 10 (1), 123-127 (1974).
- Magnuson, H. J., Eagle, H. The minimal infectious inoculum of Spirochaeta pallida (Nichols strain), and a consideration of its rate of multiplication in vivo. Am J Syph. 32, 1-18 (1948).
- Cumberland, M. C., Turner, T. B. The rate of multiplication of Treponema pallidum in normal and immune rabbits. Am J Syph. 33, 201-211 (1949).
- Norris, S. J., Edmondson, D. G. Factors affecting the multiplication and subculture of Treponema pallidum subsp. pallidum in a tissue culture system. Infect Immun. 53, 534-539 (1987).
- Baseman, J. B., Nichols, J. C., Rumpp, O., Hayes, N. S. Purification of Treponema pallidum from infected rabbit tissue: resolution into two treponemal populations. Infect Immun. 10, 1062-1067 (1974).
- Hanff, P. A., Norris, S. J., Lovett, M. A., Miller, J. N. Purification of Treponema pallidum, Nichols strain, by Percoll density gradient centrifugation. Sex Transm Dis. 11, 275-286 (1984).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved