Zum Anzeigen dieser Inhalte ist ein JoVE-Abonnement erforderlich. Melden Sie sich an oder starten Sie Ihre kostenlose Testversion.
Method Article
Isolierung und Kultivierung von primären Synovialmakrophagen und Fibroblasten aus murinem Arthritisgewebe
In diesem Artikel
Zusammenfassung
Die vorliegende Studie bietet ein modifiziertes Protokoll zur Isolierung von Synovialmakrophagen und Fibroblasten aus murinem entzündlichem Arthritisgewebe.
Zusammenfassung
Rheumatoide Arthritis ist eine Autoimmunerkrankung, die zu chronischen Entzündungen der Gelenke führt. Synovialmakrophagen und synoviale Fibroblasten spielen eine zentrale Rolle in der Pathogenese der rheumatoiden Arthritis. Es ist wichtig, die Funktionen beider Zellpopulationen zu verstehen, um die Mechanismen aufzudecken, die dem pathologischen Fortschreiten und der Remission bei entzündlicher Arthritis zugrunde liegen. Im Allgemeinen sollten In-vitro-Versuchsbedingungen die In-vivo-Umgebung so weit wie möglich nachahmen. Aus dem Primärgewebe gewonnene Zellen wurden in Experimenten zur Charakterisierung von Synovialfibroblasten bei Arthritis verwendet. Im Gegensatz dazu wurden in Experimenten, in denen die biologischen Funktionen von Makrophagen bei entzündlicher Arthritis untersucht wurden, Zelllinien, aus dem Knochenmark stammende Makrophagen und aus Blutmonozyten gewonnene Makrophagen verwendet. Es ist jedoch unklar, ob solche Makrophagen tatsächlich die Funktionen von geweberesidenten Makrophagen widerspiegeln. Um residente Makrophagen zu erhalten, wurden frühere Protokolle modifiziert, um sowohl primäre Makrophagen als auch Fibroblasten aus Synovialgewebe in einem Mausmodell für entzündliche Arthritis zu isolieren und zu expandieren. Diese primären Synovialzellen können für die In-vitro-Analyse von entzündlicher Arthritis nützlich sein.
Einleitung
Rheumatoide Arthritis (RA) ist eine Autoimmunerkrankung, die durch eine Hyperplasie der Synovialfunktion gekennzeichnet ist und zur Zerstörung der Gelenke führt 1,2. Geweberesidente Makrophagen und Fibroblasten sind in gesunden Synovialen vorhanden, um die gemeinsame Homöostase aufrechtzuerhalten. Bei RA-Patienten vermehren sich synoviale Fibroblasten (SFs), und Immunzellen, einschließlich Monozyten, infiltrieren in die Synovial- und Gelenkflüssigkeit, Prozesse, die mit Entzündungen verbundensind 1,3,4. Synoviale Makrophagen (SMs), zu denen residente Makrophagen und von Monozyten im peripheren Blut abgeleitete Makrophagen gehören, und SFs sind aberrant aktiviert und spielen eine wichtige Rolle in der RA-Pathogenese. Neuere Studien deuten darauf hin, dass Zell-Zell-Interaktionen zwischen SMs und SFs sowohl zur Exazerbation als auch zur Remission von RA 5,6 beitragen.
Um die RA-Pathogenese zu verstehen, wurden mehrere Nagetiermodelle der entzündlichen Arthritis verwendet, darunter K/BxN-Serumtransfer-Arthritis, Kollagen-induzierte Arthritis und Kollagen-Antikörper-induzierte Arthritis. Zellbasierte Assays sind in der Regel erforderlich, um molekulare Funktionen bei Arthritis zu klären. Daher wurden Primärzellen aus Tiermodellen der Arthritis isoliert. Die Methode zur Isolierung von SFs aus murinem Arthritisgewebe ist gut etabliert, und diese Zellen haben zur Aufklärung der molekularen Mechanismen in der Arthritis-Pathogenese beigetragen 7,8. Auf der anderen Seite wurden Makrophagen aus dem Knochenmark, aus Blutmonozyten gewonnene Makrophagen und Makrophagenzelllinien häufig als Makrophagenressourcen für Arthritisstudien verwendet 9,10. Da Makrophagen Funktionen erwerben können, die mit ihrer Mikroumgebung verbunden sind, fehlen den allgemeinen Quellen von Makrophagen möglicherweise die spezifischen Reaktionen auf Arthritisgewebe. Darüber hinaus ist es schwierig, durch Sortierung genügend Synovialzellen zu erhalten, da die murine Synovialflüssigkeit selbst in Arthritismodellen ein sehr kleines Gewebe ist. Die mangelnde Verwendung von Synovialmakrophagen für In-vitro-Studien war eine Einschränkung in Arthritisstudien. Die Etablierung eines Protokolls zur Isolierung und Expansion von Synovialmakrophagen wäre ein Vorteil für die Aufklärung pathologischer Mechanismen bei RA.
Bei der vorherigen Methode zur Isolierung von SFs wurden SMs verworfen7. Darüber hinaus wurde über eine Methode zur Isolierung und Expansion residenter Makrophagen aus einigen Organen berichtet11. Daher wurden bestehende Protokolle in Kombination modifiziert. Die Modifikation zielt darauf ab, die Primärkultur sowohl von SMs als auch von SFs mit hoher Reinheit zu erreichen. Das übergeordnete Ziel dieser Methode ist es, sowohl SMs als auch SFs aus murinem Arthritisgewebe zu isolieren und zu expandieren.
Protokoll
Tierversuche wurden vom Tierversuchskomitee der Universität Ehime genehmigt und in Übereinstimmung mit den Richtlinien für Tierversuche der Universität Ehime (37A1-1*16) durchgeführt.
1. Vorbereitung von Instrumenten, Reagenzien und Nährmedium
- Bereiten Sie das Nährmedium wie folgt vor: Ergänzen Sie Dulbeccos Modified Eagle Medium (DMEM) mit 10 % fötalem Kälberserum (FBS) und 1 % antibiotisch-antimykotischer Lösung (Anti-Anti).
- Bereiten Sie das Verdauungsmedium wie folgt vor: Ergänzen Sie das Nährmedium mit 1 mg/ml Kollagenase Typ IV. Passen Sie die Kollagenasekonzentration kurz vor der Anwendung an.
- Verdünnen Sie Kollagen Typ I-C auf eine Konzentration von 0,15 mg/ml mit 1 mM HCl-Lösung. Kulturschalen (Durchmesser 40 oder 60 mm) mit der verdünnten Kollagenlösung überfluten. Nach 6-12 h bei Raumtemperatur die Kollagenlösung aus dem Geschirr nehmen und bei Raumtemperatur trocknen. Die mit Kollagen überzogenen Schalen sind mindestens 1 Woche bei Raumtemperatur haltbar. Waschen Sie das vorbeschichtete Geschirr vor Gebrauch mit phosphatgepufferter Kochsalzlösung (PBS) oder Medium.
- Bereiten Sie sterile chirurgische Instrumente wie Scheren, Pinzetten mit gezackten Spitzen und Pinzetten mit feiner Spitze vor. Vor Gebrauch in 70%igem Ethanol einweichen.
2. Präparation von Synovitis-Gewebe bei Mäusen ( Abbildung 1A)
- Bereiten Sie eine Maus mit entzündlicher Arthritis in den Hinterpfoten vor.
HINWEIS: Fürdieses Protokoll wurden weibliche C57BL/6-Mäuse (18-20 g), 7-8 Wochen nach der Geburt, mit Kollagen-Antikörper-induzierter Arthritis (CAIA) oder K/BxN-Serumtransfer-Arthritis (STA) verwendet. Die Isolierung von SMs aus nicht geschwollenem (d. h. gesundem) Gewebe könnte schwierig sein, da die Anzahl der Zellen nicht ausreicht. - Betäuben Sie die Mäuse durch eine intraperitoneale Injektion von 80 mg/kg Ketamin und 16 mg/kg Xylazin. Reinigen Sie die Mäuse mit 70%igem Ethanol.
- Schneiden Sie die Brustregion mit einer Schere auf, um das Herz freizulegen. Schneiden Sie die rechte Ohrmuschel des Herzens mit einer Schere ab und stechen Sie dann eine 23-G-Schmetterlingsnadel über die Herzspitze in die linke Herzkammer, gefolgt von einem Rückfluss von 15-20 ml sterilem PBS mit einer Spritze, um peripheres Blut manuell zu entfernen (ca. 1 ml/2 s).
- Schälen Sie die Hinterpfoten, indem Sie die Haut mit einer Schere aufschneiden und die Haut mit einer Pinzette mit gezackten Spitzen herausziehen.
HINWEIS: Nach diesem Schritt wird die Verwendung einer Pinzette für die Handhabung von Proben empfohlen. Berühren Sie das geschälte Gewebe nicht mit den Fingern, um das Anhaften von Mäusehaaren zu vermeiden. - Verschieben Sie die Zehengrundgelenke durch Ziehen, gefolgt von einem Durchtrennen der Bänder der Gelenke mit einer Schere, um die Zehen zu entfernen.
- Schneide die Sehnen der Unterschenkelmuskulatur in der Nähe des Knöchels mit einer Schere durch. Fassen Sie die Sehne mit einer Pinzette und schälen Sie die Muskeln proximal im Unterschenkel ab, um das Schienbein freizulegen. Entferne das Wadenbein.
- Auskugeln des Kniegelenks durch Ziehen, anschließendes Durchtrennen der Gelenkbänder mit einer Schere, um das Schienbein mit der geschwollenen Hinterpfote zu lösen. Bewahren Sie die Proben in eiskaltem Nährmedium (0,3 ml/cm2) auf, bis Sie sie bis Schritt 3.1 verarbeiten.
3. Verdauung von Synovitis-Gewebe ( Abbildung 1B)
- Saugen Sie das Nährmedium ab, vermeiden Sie die Probe und fügen Sie dann frisches Nährmedium (0,3 ml/cm2) hinzu. Wiederholen Sie den Waschvorgang drei- bis viermal.
HINWEIS: Ab diesem Schritt sollte die Handhabung der Proben aseptisch in einem sauberen Labortisch oder einer Sicherheitswerkbank durchgeführt werden. - Verschieben Sie alle Gelenke der Proben, indem Sie mit einer feinen Pinzette im Nährmedium unter einem Stereomikroskop (bei 10-20-facher Vergrößerung) ziehen. Eine Pinzette mit feiner Spitze ist in diesem Schritt praktisch. Entferne das Schienbein und so viele Gefäße, Sehnen und Bänder wie möglich. Achte darauf, dass du dir die Knochen nicht brichst, wenn du dich verrenkst.
- Bereiten Sie zwei 15-ml-Röhrchen pro Probe vor. Übertragen Sie die ausgerenkten Knochen mit Weichteilen mit einer Pinzette in das erste 15-ml-Röhrchen. Geben Sie 4 ml Verdauungsmedium pro Probe, das aus beiden Hinterpfoten gewonnen wurde, in das Röhrchen.
- Um Restzellen und Gewebefragmente zu sammeln, wird das Medium, in dem sich die Probe befand, in das zweite 15-ml-Röhrchen überführt. Das Medium wird bei 500 x g für 5 min bei Raumtemperatur zentrifugiert. Nach dem Entfernen des Überstandes wird das Pellet mit 1 ml Verdauungsmedium resuspendiert und die Zell- und Gewebefragmentlösung in das erste 15-ml-Röhrchen überführt, das fast das gesamte Gewebe enthält (insgesamt 5 ml Verdauungsmedium/Hinterpfoten).
- Die Probe wird für 60-120 min bei 37 °C unter Schütteln in einem Hybridisierungsofen aufgeschlossen.
HINWEIS: Der optimale Zeitpunkt für die Verdauung der Proben sollte festgelegt werden. Die Zeit ist abhängig vom Grad der Schwellung in den Knöcheln und Kollagenasen. In den meisten Fällen sind 60-120 min ausreichend. Nach 60 Minuten Inkubation wird ein Teil der aufgeschlossenen Probe durch Pipettieren entnommen und unter dem Mikroskop beobachtet. Wenn die Verdauung unzureichend ist, sollte die Inkubation fortgesetzt und die Verdauung alle 30 Minuten überprüft werden. - Pipetieren Sie die Lösung gut. Die Zelllösung wird durch ein Zellsieb (40 μm Porengröße) in ein 50-ml-Röhrchen filtriert.
- Geben Sie 10 ml Nährmedium durch das Zellsieb in das 50-ml-Röhrchen. Bei 300 x g 5 min bei Raumtemperatur zentrifugieren.
- Nach dem Entfernen des Überstandes mit 10 ml Nährmedium resuspendieren. Wiederholen Sie die Zentrifugation. Nach dem Entfernen des Überstandes mit 2 ml Nährmedium resuspendieren.
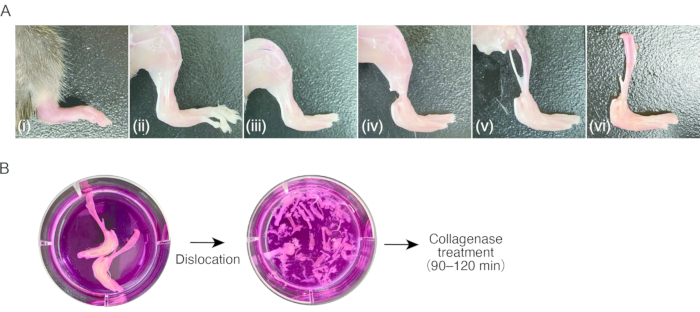
Abbildung 1: Verfahren der Probenahme von murinem Arthritisgewebe und Kollagenase-Verdauung . (A) (i) Hinterpfote der Maus mit entzündlicher Arthritis. ii) Entfernung der Haut an der Hinterpfote. (iii) Luxation der Zehengrundgelenke und Entfernung der Zehen. (iv) Durchtrennen der Sehnen im Knöchel. (v) Entfernung der Muskeln in den Unterschenkeln. (vi) Luxation des Kniegelenks. (b) links; Herausgeschnittene Beine in Nährmedium. Rechts; ausgerenkter Tarsus und Metatarsus im Nährmedium. Bitte klicken Sie hier, um eine größere Version dieser Abbildung anzuzeigen.
4. Isolierung von synovialen Fibroblasten ( Abbildung 2A)
- Säen Sie alle Zellsuspensionen, die Sie von beiden Knöcheln erhalten haben, auf die kollagenbeschichtete Schale.
HINWEIS: Wenn Sie Knöchel mit schwacher oder mäßiger Schwellung verwenden, um die Zellen zu erhalten, wird eine Schale mit einem Durchmesser von 40 mm angewendet. Die mit Kollagen beschichtete Schalengröße kann auf eine Schale mit einem Durchmesser von 60 mm geändert werden, wenn beide Knöchel stark geschwellt sind. - Nährmedium (ca. 222 μl/cm2) hinzufügen. 1 h bei 37 °C in befeuchteter Atmosphäre mit 5 % CO 2 inkubieren.
- Sammeln Sie nicht adhärente Zellen mit einer Pipette (Verwendung in Schritt 5.1). Waschen Sie die mit Kollagen überzogene Schale mit Nährmedium und sammeln Sie das Medium. Kultivieren Sie die adhärenten Zellen in frischem Medium (Abbildung 2B,i). Die meisten Zellen, die schnell an der kollagenbeschichteten Schale haften, weisen eine fibroblastoide (spindelförmige) Morphologie auf.
- Passage subkonfluenter Zellen durch Behandlung mit 0,05 % Trypsin in Hanks' ausgewogener Salzlösung (HBSS). Bei dieser Methode ist die Kontamination anderer Zellen begrenzt, wenn auch in der anfänglichen Expansion. Wenn mehr gereinigte fibroblastenähnliche Zellen benötigt werden, führen Sie eine wiederholte Passage durch, um die Reinheit zu verbessern. Es wird jedoch auch die Ausdehnung des Zytoplasmas der Zellen bei Adhäsion beobachtet (Abbildung 2B,ii). Da übermäßige Passagen den Verlust naiver Eigenschaften in den Zellen beeinflussen, verwenden Sie Zellen mit weniger als 5 Passagen.
5. Isolierung von Synovialmakrophagen ( Abbildung 2A)
- Säen Sie alle nicht adhärenten Zellen aus Schritt 4.4 auf Schalen (Durchmesser von 40 oder 60 mm), die nicht mit Kollagen beschichtet wurden.
HINWEIS: Zu den nicht adhärenten Zellen gehören Makrophagen, andere Lymphozyten und Restfibroblasten aus Synovitisgewebe. - Die Bulk-Zellen werden 1 Tag lang bei 37 °C in einer befeuchteten Atmosphäre mit 5 % CO2 kultiviert.
- Um nicht adhärente Lymphozyten zu entfernen, saugen Sie das Nährmedium ab und fügen Sie dann frisches Nährmedium hinzu. Wiederholen Sie diesen Vorgang zwei- bis dreimal (Abbildung 2B,iii).
- Die adhärenten Bulk-Zellen werden 1-2 Wochen lang in einem Kulturmedium kultiviert, wobei das Medium alle 2 Tage gewechselt wird, wobei die Konfluenz beibehalten wird (Abbildung 2B,iv).
HINWEIS: Die Anzahl der SMs nimmt unter Co-Kulturbedingungen mit SFs langsam zu. Daher sollte die Co-Kulturperiode nach Bedarf angepasst werden. - Zweimal mit PBS oder HBSS waschen. Mit 0,05 % Trypsin in HBSS (ca. 55 μl/cm2) für 3 min bei 37 °C in einer befeuchteten Atmosphäre mit 5 % CO2 behandeln. Fibroblasten lösen sich durch Trypsinbehandlung leicht von der Kulturschale, und Makrophagen zeigen eine Resistenz gegen die Trypsinbehandlung. Verwenden Sie diese Eigenschaft für die Auswahl von Synovialmakrophagen.
- Das Nährmedium (ca. 222 μl/cm2) wird vorsichtig zu 0,05 % Trypsin in HBSS gegeben. Gießen Sie das Medium nach diesem Schritt nicht mehr direkt auf die Zellen.
- Um abgelöste Zellen zu entfernen, saugen Sie das Kulturmedium ab und fügen Sie dann vorsichtig frisches Nährmedium hinzu. Wiederholen Sie diesen Vorgang zwei- bis dreimal. Die verbleibenden Zellen bleiben bis zur Verwendung auf der Schale in frischem Nährmedium (Abbildung 2B,v).
HINWEIS: Nach einer Trypsin-Behandlung weisen adhärente Zellen makrophagenähnliche morphologische Merkmale auf.

Abbildung 2: Trennung von makrophagenreichen und fibroblastenreichen Fraktionen aus entzündlichem Arthritisgewebe. (A) Schema des Verfahrens zur Trennung von makrophagenreichen und fibroblastenreichen Zellen aus Arthritisgewebe. (B) Repräsentative Phasenkontrastbilder der Phasen des Verfahrens, (i) bis (v) in Abbildung 2A. Der Maßstabsbalken stellt 100 μm dar. Bitte klicken Sie hier, um eine größere Version dieser Abbildung anzuzeigen.
Ergebnisse
Weibliche C57BL/6-Mäuse im Alter von 7-8 Wochen erlitten eine Kollagen-Antikörper-induzierte Arthritis. Makrophagen-ähnliche Zellen und Fibroblasten-ähnliche Zellen wurden unabhängig voneinander aus entzündlichem Arthritisgewebe gemäß dem oben beschriebenen Verfahren isoliert (Abbildung 2A,B). Makrophagen-ähnliche Zellen wurden unmittelbar nach Schritt 5.7 verwendet. Fibroblasten-ähnliche Zellen wurden nach Schritt 4.4 zunächst subkonfluent kultiviert und dann in ...
Diskussion
Diese hier entwickelte Methode verbessert frühere Techniken zur Isolierung sowohl von SFs aus muriner Arthritis als auch residenter Makrophagen aus einer Reihe von Organen 7,11. Die modifizierte Methode kann sowohl Makrophagen als auch Fibroblasten mit hoher Reinheit aus entzündlichen Synovialen isolieren, ist einfach und reproduzierbar. Da die Methode keine komplexen Instrumente wie einen Zellsortierer benötigt, kann sie von jedem durchgeführt werden. Darüb...
Offenlegungen
Die Autoren erklären, dass sie keine konkurrierenden Interessen haben.
Danksagungen
Die Autoren danken den Mitarbeitern der Abteilung für medizinische Forschungsunterstützung, dem Advanced Research Support Center (ADRES) und den Mitgliedern der Abteilung für integrative Pathophysiologie, Proteo-Science Center (PROS) der Universität Ehime, für ihre technische und hilfreiche Unterstützung. Diese Studie wurde teilweise von der Japan Society for the Promotion of Science (JSPS) KAKENHI-Stipendien JP17K17929, JP19K16015, JP21K05974 (an NS) und JP23689066, JP15H04961, JP15K15552, JP17K19728, JP19H03786 (an YI) unterstützt. Stipendien der Osaka Medical Research Foundation for Intractable Diseases, der Nakatomi Foundation, des Rising Stars Grant der Japanese Society for Bone and Mineral Research (JSBMR), der Sumitomo Foundation, der SENSHIN Medical Research Foundation, der Mochida Memorial Foundation (an NS); und ein Stipendium der Takeda Science Foundation für medizinische Forschung, ein Projektstipendium der UCB Japan (UCBJ) und das JSBMR Frontier Scientist Stipendium 2019 (für YI).
Materialien
| Name | Company | Catalog Number | Comments |
| 5.0 g/L Trypsin/5.3 mmol/L EDTA solution | nacalai tesque | 35556-44 | Diluted with HBSS |
| Antibiotic–antimycotic (anti/anti) | Gibco | 15240-062 | |
| Butterfly needle | TERUMO | SV-23DLK | 23G |
| Cell strainer | Falcon | 352340 | 40 µm pore, Nylon |
| Cellmatrix Type I-C | Nitta gelatin | 637-00773 | Type I-C collagen |
| Centriguge tube 15 | TPP | 91014 | 15 mL tube |
| Centriguge tube 50 | TPP | 91050 | 50 mL tube |
| Collagenase from C. Histolyticum | Sigma | C5138 | Type IV collagenase |
| Dulbecco’s Modified Eagle Medium GlutaMax (DMEM) | Gibco | 10569-010 | |
| Fetal bovine serum (FBS) | SIGAM | 173012 | Heat inactivation was performed |
| Hanks' balanced salt solution (HBSS) | Wako | 085-09355 | |
| Scissors | Bio Research Center | PRI28-1525A | |
| Tissue culture dish 40 | TPP | 93040 | For cell culture |
| Tissue culture dish 60 | TPP | 92006 | For cell culture |
| Tweezers | KFI | 1-9749-31 | Fine-point |
| Tweezers | Bio Research Center | PRI28-1522 | Serrated tip |
| ZEISS Stemi 305 | ZEISS | STEMI305-EDU | Stereomicroscope |
Referenzen
- Smolen, J. S., Aletaha, D., McInnes, I. B. Rheumatoid arthritis. Lancet. 388 (10055), 2023-2038 (2016).
- McInnes, I. B., Schett, G. The pathogenesis of rheumatoid arthritis. The New England Journal of Medicine. 365 (23), 2205-2219 (2011).
- Kurowska-Stolarska, M., Alivernini, S. Synovial tissue macrophages: friend or foe. RMD Open. 3 (2), (2017).
- Hannemann, N., Apparailly, F., Courties, G. Synovial macrophages: from ordinary eaters to extraordinary multitaskers. Trends in Immunology. 42 (5), 368-371 (2021).
- Alivernini, S., et al. Distinct synovial tissue macrophage subsets regulate inflammation and remission in rheumatoid arthritis. Nature Medicine. 26 (8), 1295-1306 (2020).
- Saeki, N., Imai, Y. Reprogramming of synovial macrophage metabolism by synovial fibroblasts under inflammatory conditions. Cell Communication and Signaling. 18 (1), 188 (2020).
- Armaka, M., Gkretsi, V., Kontoyiannis, D., Kollias, G. A standardized protocol for the isolation and culture of normal and arthritogenic murine synovial fibroblasts. Protocol Exchange. , (2009).
- Saeki, N., et al. Epigenetic regulator UHRF1 orchestrates proinflammatory gene expression in rheumatoid arthritis in a suppressive manner. The Journal of Clinical Investigation. 132 (11), (2022).
- Midwood, K., et al. Tenascin-C is an endogenous activator of Toll-like receptor 4 that is essential for maintaining inflammation in arthritic joint disease. Nature Medicine. 15 (7), 774-780 (2009).
- You, D. G., et al. Metabolically engineered stem cell-derived exosomes to regulate macrophage heterogeneity in rheumatoid arthritis. Science Advances. 7 (23), 0083 (2021).
- Ogawa, K., Tsurutani, M., Hashimoto, A., Soeda, M. Simple propagation method for resident macrophages by co-culture and subculture, and their isolation from various organs. BMC Immunology. 20 (1), 34 (2019).
- Andrä, I., et al. An evaluation of T-cell functionality after flow cytometry sorting revealed p38 MAPK activation. Cytometry Part A. 97 (2), 171-183 (2020).
- Ryan, K., Rose, R. E., Jones, D. R., Lopez, P. A. Sheath fluid impacts the depletion of cellular metabolites in cells afflicted by sorting induced cellular stress (SICS). Cytometry Part A. 99 (9), 921-929 (2021).
- Llorente, I., García-Castañeda, N., Valero, C., González-Álvaro, I., Castañeda, S. Osteoporosis in rheumatoid arthritis: dangerous liaisons. Frontiers in Medicine. 7, 601618 (2020).
- Croft, A. P., et al. Distinct fibroblast subsets drive inflammation and damage in arthritis. Nature. 570 (7760), 246-251 (2019).
- Wei, K., et al. Notch signalling drives synovial fibroblast identity and arthritis pathology. Nature. 582 (7811), 259-264 (2020).
Nachdrucke und Genehmigungen
Genehmigung beantragen, um den Text oder die Abbildungen dieses JoVE-Artikels zu verwenden
Genehmigung beantragenThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Alle Rechte vorbehalten