Se requiere una suscripción a JoVE para ver este contenido. Inicie sesión o comience su prueba gratuita.
Method Article
Agroinfiltración forzada al vacío para la transformación in planta de plantas recalcitrantes: el cacao como caso de estudio
En este artículo
Resumen
En este trabajo presentamos el primer protocolo de infiltración localizada al vacío para estudios in vivo de la transformación genética de plantas de gran tamaño. Utilizando esta metodología, logramos por primera vez la transformación transitoria del cacao mediada por Agrobacterium in planta .
Resumen
La transformación transitoria en planta es una alternativa rápida y rentable para la transformación genética de plantas. La mayoría de los protocolos para la transformación in planta se basan en el uso de la transformación mediada por Agrobacterium. Sin embargo, los protocolos actualmente en uso están estandarizados para plantas de pequeño tamaño debido a las limitaciones físicas y económicas de someter plantas de gran tamaño a un tratamiento de vacío. En este trabajo se presenta un protocolo efectivo para la agroinfiltración localizada al vacío personalizada para plantas de gran tamaño. Para evaluar la eficacia del método propuesto, probamos su uso en plantas de cacao, una especie de planta tropical recalcitrante a la transformación genética. Nuestro protocolo permitió aplicar hasta 0,07 MPa de vacío, con repeticiones, a una parte aérea localizada de las hojas de cacao, posibilitando forzar la infiltración de Agrobacterium en los espacios intercelulares de las hojas adheridas. Como resultado, logramos la transformación transitoria mediada por Agrobacterium en la planta de las hojas de cacao adheridas que se expresan para el sistema reportero RUBY. Esta es también la primera transformación transitoria del cacao mediada por Agrobacterium. Este protocolo permitiría la aplicación del método de agroinfiltración al vacío a otras especies de plantas con limitaciones de tamaño similares y abriría la puerta a la caracterización in planta de genes en especies leñosas recalcitrantes de gran tamaño.
Introducción
Los métodos de transformación genética de las plantas son esenciales para probar las funciones biológicas de los genes y son especialmente útiles hoy en día, dado el gran número de genes no caracterizados que se predijeron en la era postgenómica1. Estos métodos se pueden utilizar para obtener líneas completamente transformadas o para expresar genes de forma transitoria. La transformación estable ocurre cuando el ADN extraño que el huésped ha absorbido se integra completa e irreversiblemente en el genoma del huésped, y las modificaciones genéticas se transmiten a las generaciones posteriores. La expresión transitoria, conocida como transformación transitoria, se produce a partir de las múltiples copias de ADN-T transferidas por Agrobacterium a la célula, que no se han integrado en el genoma del huésped, y alcanza su punto máximo entre 2 y 4 días después dela infección.
Vale la pena señalar que los ensayos de expresión transitoria suelen ser suficientes para la caracterización funcional de los genes y pueden ofrecer varias ventajas sobre la transformación estable. Por ejemplo, la transformación transitoria no requiere procedimientos de regeneración basados en cultivos de tejidos. Otra ventaja es que es compatible con el análisis funcional in planta de genes, existiendo varios ejemplos exitosos de protocolos bien estandarizados para especies de plantas modelo, como Arabidopsis thaliana3 y Nicotiana benthamiana4, pero aún limitado en especies no modelo5.
El desarrollo de ensayos transitorios depende de la disponibilidad de métodos eficientes de transferencia de genes. Para ello, los enfoques más populares se basan en la infiltración de Agrobacterium , que aprovecha la capacidad única de Agrobacterium para transferir ADN a las células vegetales6. Otra herramienta útil para estos análisis es el uso de genes reporteros, como las proteínas fluorescentes verdes (GFP), la β-glucuronidasa (GUS), la luciferasa o el RUBY, todos los cuales se emplean para rastrear eventos de transformación. Entre estos sistemas reporteros, RUBY es actualmente el más fácil de visualizar y se basa en la conversión de tirosina en betalaínas a través de tres reacciones enzimáticas escalonadas. A diferencia de otros sistemas reporteros, las betalaínas resultantes se pueden observar fácilmente como pigmentos de colores brillantes en el tejido vegetal transformado sin necesidad de equipos sofisticados o reactivos adicionales7.
Cuando se infiltra una suspensión de Agrobacterium en el espacio intercelular del mesófilo de la hoja, el paso más crítico para el éxito de la agroinfección es superar la barrera física impuesta por la cutícula epidérmica de las hojas8. Mientras que para algunas plantas, un gradiente de presión creado con una jeringa sin aguja (jeringa Agroinfiltración) es suficiente para una agroinfiltración eficiente, como ocurre en Nicotiana benthamiana9, otras especies de plantas pueden requerir un gradiente de presión mayor como el creado con la ayuda de bombas de vacío10. En los procesos asistidos por vacío, la agroinfiltración se produce en dos pasos. En el primero, el vacío sirve para someter el material vegetal a una presión reducida, forzando la liberación de gases de los espacios aéreos del mesófilo a través de los estomas y las heridas. Luego, durante una fase de represurización, la suspensión de Agrobacterium se infiltra en los espacios intercelulares a través de los estomas y las heridas11.
En comparación con la infiltración de jeringas, la infiltración al vacío permite una mayor frecuencia de uso, repetibilidad y la capacidad de controlar la presión y la duración en cada etapa del proceso de infiltración10. En hojas de diferentes especies vegetales como la espinaca (Spinacia oleracea)12, la peonía (una planta leñosa perenne) (Paeonia ostii)13 y el caupí (Vigna unguiculata)14, los protocolos de agroinfiltración al vacío lograron una tasa de infiltración más profunda que la infiltración con jeringa. De manera similar, en tomate (Lycopersicon esculentum)15 y gerbera (Gerbera hybrida)16, la agroinfiltración al vacío produjo un silenciamiento génico más fuerte y uniforme que la infiltración con jeringa. Una ventaja adicional de la infiltración al vacío es la menor dependencia del genotipo, en comparación con la infiltración de jeringas, que se observó recientemente en tres variedades de cítricos (Fortunella obovata, Citrus limon y C. grandis)17. Sin embargo, cuando se trata de aplicar la agroinfiltración al vacío a plantas que son demasiado grandes para caber en los desecadores, el tamaño de las cámaras de vacío puede ser una limitación, como suele ocurrir con las plantas leñosas tropicales.
A continuación, describimos un protocolo que supera la limitación espacial de las cámaras de vacío, probando su utilidad para la transformación transitoria in planta de las hojas de cacao. Presentamos el primer método de infiltración al vacío localizado para cacao, que no requiere equipo adicional e incluso permite el uso de los mismos desecadores de laboratorio utilizados para la infiltración de toda la planta, pero con una adaptación sencilla que permite el acceso de una parte de la planta dentro de la cámara de vacío, permitiendo su uso en diferentes etapas de desarrollo de la planta. Para probar la utilidad del método de infiltración al vacío localizado propuesto, seleccionamos el cacao como proxy de una especie de planta tropical de hoja grande que es difícil de transformar. Utilizando este método de infiltración localizada, recientemente reportamos la primera expresión transitoria in planta en aguacate por infiltración al vacío mediada por Agrobacterium con condiciones previamente optimizadas para hojas separadas18, y aquí reportamos la primera expresión transitoria in planta en cacao.
Protocolo
1. Cultivo de Agrobacterium tumefaciens
- Descongelación de células electrocompetentes de Agrobacterium tumefaciens cepa LBA4404.
- Agregue 1 mL de malta de levadura (YM; Tabla 1) caldo a un tubo de cultivo de 17 mm x 100 mm. Guarde este tubo para más tarde y manténgalo a temperatura ambiente (RT).
- En un tubo de microfuga de 1,5 ml, agregue 30 μl de las células de Agrobacterium descongeladas y 100-250 ng (hasta 5 μl) del ADN que contiene 35S:RUBY. Mezclar suavemente.
NOTA: El 35S:RUBY fue un regalo de Yunde Zhao. Para evitar la formación de arcos en la muestra, reduzca la presencia de compuestos iónicos tanto como sea posible. Estos compuestos iónicos pueden ser sales residuales de la precipitación de etanol del ADN19. - En este punto, coloque una cubeta de electroporación de 1 mm sobre hielo.
- Transfiera la mezcla de suspensión anterior a una cubeta de electroporación refrigerada de 1 mm. Mantén todo en hielo. Limpie los electrodos metálicos de la cubeta.
- Ajuste el electroporador a Agr (2,2 kV, ~5 ms, 1 pulso). Coloque la cubeta dentro de la cámara de electroporación.
- Presione el botón Pulse . Registre los parámetros de impulso. Si la muestra se arqueó, el proceso de electroporación falló.
NOTA: Es fundamental transferir rápidamente las células al caldo YM justo después del pulso. Retrasar esta transferencia puede reducir drásticamente la eficiencia de la transformación20. - Inmediatamente, use el caldo YM guardado para transferir las celdas de la cubeta al tubo de 17 mm x 100 mm. Vuelva a suspender las células suavemente.
- Incubar las células transformadas durante 3 h a 28 °C y 250 rpm en una incubadora orbital.
NOTA: Este cultivo no contiene antibióticos; Tenga cuidado con las condiciones asépticas adecuadas. - Enrasar este cultivo en placas selectivas de agar YM21. Para la cepa transformada LBA4404-RUBY, asegúrese de que estas placas contengan rifampicina (25 μg/mL), espectinomicina (50 μg/mL) y estreptomicina (50 μg/mL). Incubar esta placa durante la noche en una incubadora de pie a 28 °C.
NOTA: En este protocolo se utilizó el vector 35S:RUBY , que confiere resistencia bacteriana a la espectinomicina (50 μg/mL) y funciona como reportero visual sobre el tejido vegetal infiltrado. - Inocular las colonias del cultivo nocturno en 12,5 mL de una mezcla de caldo YM y caldo Luria Bertani (LB) (en proporción 9:1, respectivamente), 10 mM de MES, pH 5,722. Asegúrese de que este medio líquido selectivo contenga los mismos antibióticos utilizados en el paso 1.10. Consulte la Tabla 1 para ver los ingredientes y las concentraciones de estos medios.
- Al incubar Agrobacterium, deje suficiente espacio de aireación para el cultivo, aproximadamente de 4 a 5 veces el volumen de líquido. Use caldo YM para la LBA4404 de cepas de Agrobacterium para evitar la aglomeración de células23.
- Incubar el cultivo durante 16 h a 250 rpm en una incubadora orbital a 28 °C.
- Escale el cultivo hasta 10 veces el volumen inicial con el mismo medio utilizado en el paso 1.11.
- Incubar el cultivo durante 16 h y 250 rpm en una incubadora orbital a 28 °C.
- Ajuste el cultivo durante la noche a una densidad óptica (OD600) de 0,4. Añadir 20 μM de acetosiringona (AS).
- Incubar a 250 rpm en una incubadora orbital a 28 °C hasta que el diámetro exterior600 alcance aproximadamente 1,0.
- Centrifugar las células a 4 500 x g durante 10 min a 20 °C.
- Vuelva a suspender el gránulo con solución de suspensión (10 mM MES, 10 mM MgCl2, pH 5,7), ajustando el OD600 a 0,6. Añadir 200 μM AS24.
NOTA: Preincubar la solución de suspensión a 28 °C. Si la solución de suspensión está fría cuando se agrega, las células precipitarán. - Dejar la suspensión bacteriana durante 2-24 h en condiciones de oscuridad y 25 °C. No se requiere agitación22.
2. Selección de plantas
- Elija una planta con una rama con hojas en la etapa óptima para la agroinfiltración.
NOTA: La planta puede ser adulta o un árbol maduro. Para el cacao, se recomiendan hojas tiernas de etapa C. Estas hojas son de color bronce a verde claro; no están completamente expandidos ni son tan rígidos como las hojas de la etapaD 25 (Figura 1).- Como control, realizar simultáneamente la agroinfiltración en otras plantas (por ejemplo, Nicotiana tabacum) con una alta eficiencia de agroinfiltración que se reporta para la cepa y el vector utilizados.
NOTA: Si no se obtienen resultados positivos en este control, es posible que los resultados negativos se deban a la deformación o al vector utilizado.
- Como control, realizar simultáneamente la agroinfiltración en otras plantas (por ejemplo, Nicotiana tabacum) con una alta eficiencia de agroinfiltración que se reporta para la cepa y el vector utilizados.
3. Configuración de la cámara de vacío
- Como cámara de vacío, use un desecador que tenga un medidor de vacío para medir la presión de vacío en el interior.
- Añadir 250 μM de ácido jasmónico (AJ)18,26 a la suspensión de Agrobacterium a partir del paso 1.19.
- Transfiera el cultivo de Agrobacterium a un vaso de precipitados de boca ancha para sumergir la rama y las hojas seleccionadas. A continuación, coloque el vaso de precipitados con cultivo de Agrobacterium dentro del desecador.
- Coloque la rama entre el desecador y su tapa. Asegúrese de sumergir las hojas deseadas dentro del cultivo de Agrobacterium . A continuación, use un anillo de salto, que es un anillo redondo con un recorte que permite que la rama de la planta ingrese al desecador. El anillo de salto también actúa como un espaciador entre la parte inferior y superior del desecador.
- Asegúrese de que la junta sea lo suficientemente estable estructuralmente como para evitar que se aplaste con la tapa, lo suficientemente flexible como para doblarse y ajustarse a la circunferencia del desecador y no esté hecha de un material poroso.
NOTA: Este estudio utilizó un alambre de metal trenzado que consiste en varios alambres más pequeños trenzados juntos, recubiertos con un material plástico no poroso que se asemeja a una junta (Figura 2). - Para fijar la rama en el desecador, utilice material de impresión de silicona. Asegúrese de que el material sea pegajoso, no poroso, químicamente inerte al desecador y a la planta, y fácil de aplicar y rellene los pequeños huecos entre la rama, la junta y el desecador.
- Una vez que el material de impresión de silicona se polimerice (esto tarda aproximadamente 1 minuto) y fije la rama en su lugar, cierre el desecador. Asegúrate de no dejar huecos.
- Conecte el desecador a la bomba de vacío (Figura 3).
4. Infiltración al vacío
- Ponga en marcha la bomba de vacío hasta que alcance -0,07 MPa.
- Una vez que alcance esta presión, cierre la válvula de presión y apague la bomba de vacío. Mantenga esta presión durante 5 min.
- Abra la válvula de presión para restablecer la presión de la cámara.
NOTA: Este es un paso crítico. Restaure la presión de la cámara de forma gradual y constante. Puede tomar hasta 3 minutos volver a presurizar completamente el desecador. La represurización prolongada aumenta el número de bacterias infiltradas en el interior del tejido10. - Repite este proceso dos veces más.
- Retire la rama de la suspensión celular y el desecador.
- Limpiar las hojas infiltradas con agua destilada.
5. Incubación de hojas infiltradas
- Deje que las hojas infiltradas permanezcan en condiciones de oscuridad a 25 °C durante 48 h.
- A continuación, exponga el tejido infiltrado a un fotoperiodo de luz/oscuridad de 16/8 h.
- Evalúe la transformación transitoria de la hoja 3-7 días después de la infección (DPI).

Figura 1: Etapas de desarrollo de las hojas de cacao. (A-E) Etapas de desarrollo25. Haga clic aquí para ver una versión más grande de esta figura.
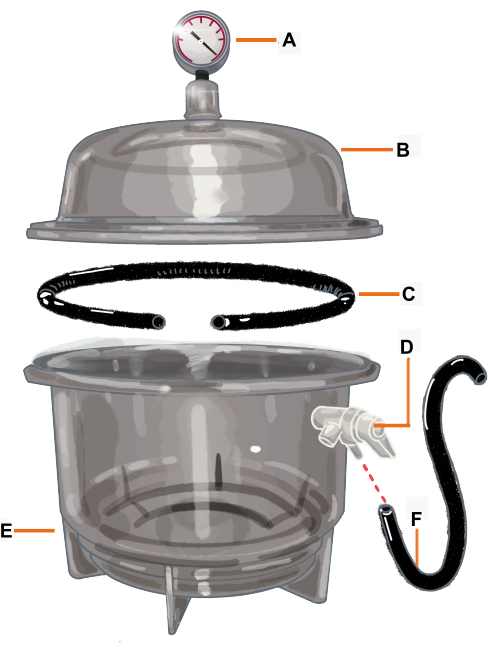
Figura 2: Configuración de la cámara de vacío y sus componentes. La cámara de vacío es un desecador conectado a un vacuómetro. La junta / junta tórica se corta para que tenga una abertura donde se colocará la rama. (A) Vacuómetro, (B) Tapa, (C) Junta/junta tórica, (D) Válvula de presión, (E) Desecador, (F) Manguera. Haga clic aquí para ver una versión más grande de esta figura.

Figura 3: Sistema de agroinfiltración al vacío en planta . Para evitar pérdidas de vacío durante el proceso de infiltración, es fundamental asegurar la rama al desecador y la junta/junta tórica con material de impresión de silicona. (A) Planta de cacao, (B) Cámara de vacío, (C) Material de impresión de silicona, (D) Hojas sumergidas en suspensión de Agrobacterium , (E) Bomba de vacío. Haga clic aquí para ver una versión más grande de esta figura.
Resultados
Este protocolo presenta un método eficaz de agroinfiltración para plantas leñosas de gran tamaño. Con este protocolo, logramos alcanzar una presión de vacío de -0.07 MPa, lo que resultó en la infiltración efectiva y localizada de las hojas de cacao. En la Figura 4, observamos el proceso de configuración del sistema de infiltración, y en la Figura 5, la configuración final.
Discusión
En este trabajo, presentamos un protocolo de agroinfiltración eficiente y de bajo costo para la transformación transitoria in planta de plantas leñosas, utilizando como ejemplo las plantas de cacao. Dada la conocida restricción que representa la cutícula de las hojas para la transformación de los tejidos vegetales, nos concentramos en desarrollar una estrategia para facilitar la agroinfiltración por vacío en plantas leñosas, que suelen ser recalcitrantes a este procedimiento.
Divulgaciones
Los autores no tienen ningún conflicto de intereses que declarar.
Agradecimientos
Agradecemos al Lic. Jesús Fuentes González y Néstor Iván Robles Olivares por su ayuda en la filmación del video. Agradecemos las generosas donaciones de la Dra. Antonia Gutiérrez Mora del CIATEJ (Theobroma cacao plants). También agradecemos al CIATEJ y al Laboratorio Nacional PlanTECC, México, por el apoyo de las instalaciones. H.E.H.D. (CVU: 1135375) realizó estudios de maestría con financiamiento del Consejo Nacional de Humanidades, Ciencia y Tecnología, México (CONAHCYT). R.U.L. agradece el apoyo del Consejo Estatal de Ciencia y Tecnología de Jalisco (COECYTJAL) y de la Secretaría de Innovación, Ciencia y Tecnología (SICYT), Jalisco, México (Subvención 7270-2018).
Materiales
| Name | Company | Catalog Number | Comments |
| 35S:RUBY plasmid | Addgene | 160908 | http://n2t.net/addgene:160908 ; RRID:Addgene_160908 |
| 1 mm electroporation cuvette | Thermo Fisher Scientific | FB101 | Fisherbrand Electroporation Cuvettes Plus |
| Desiccator | Bel-Art SP SCIENCEWARWE | F42400-2121 | |
| Freeze dryer | LABCONCO | 700402040 | |
| K2HPO4 | Sigma Aldrich | P8281-500G | For YM medium add 0.38 g/L |
| LBA4404 ElectroCompetent Agrobacterium | Intact Genomics USA | 1285-12 | https://intactgenomics.com/product/lba4404-electrocompetent-agrobacterium/ |
| Mannitol | Sigma Aldrich | 63560-250G-F | For YM medium add 10 g/L |
| MES | Sigma Aldrich | PHG0003 | (For LB, YM and resuspension medium) add 1.95 g/L (10mM) |
| MgCl2 | Sigma Aldrich | M8266 | For resuspension medium add 0.952 g/L (10 mM) |
| MgSO4·7H20 | Sigma Aldrich | 63138-1KG | For YM medium add 0.204 g/L |
| MicroPulser Electroporation Apparatus | Biorad | 165-2100 | |
| NaCl | Karal | 60552 | For LB medium add 5 g/L; For YM medium add 0.1 g/L |
| NanoDrop One Microvolume UV-Vis Spectrophotometer | Thermo Fisher Scientific | 13-400-518 | |
| President Silicone Impression material | COLTENE | 60019938 | |
| Rifampicin | Gold-Bio | R-120-1 | (100 mg/mL) |
| Silicone Impression material gun | Andent | TBT06 | |
| Spectinomycin | Gold-Bio | S-140-SL10 | (100 mg/mL) |
| Streptomycin | Gold-Bio | S-150-SL10 | (100 mg/mL) |
| Tryptone enzymatic digest from casein | Sigma Aldrich | 95039-1KG-F | For LB medium add 10 g/L |
| Yeast extract | MCD LAB | 9031 | For LB medium add 5 g/L; For YM medium add 0.4 g/L |
Referencias
- Yang, J., Jia, M., Guo, J., Huang, L. Q. Functional Genome of Medicinal Plants. Molecular Pharmacognosy. , (2019).
- Janssen, B. J., Gardner, R. C. Localized transient expression of GUS in leaf discs following cocultivation with Agrobacterium. Plant Molecular Biology. 14 (1), 61-72 (1990).
- Wang, X., et al. Identification and functional analysis of in vivo phosphorylation sites of the Arabidopsis BRASSINOSTEROID-INSENSITIVE1 receptor kinase. Plant Cell. 17 (6), 1685-1703 (2005).
- Pan, Z., et al. In vivo assembly of the sorgoleone biosynthetic pathway and its impact on agroinfiltrated leaves of Nicotiana benthamiana. The New Phytologist. 230 (2), 683-697 (2021).
- Manavella, P. A., Chan, R. L. Transient transformation of sunflower leaf discs via an agrobacterium-mediated method: Applications for gene expression and silencing studies. Nature Protocols. 4 (11), 1699-1707 (2009).
- Guo, M., Ye, J., Gao, D., Xu, N., Yang, J. Agrobacterium-mediated horizontal gene transfer: Mechanism, biotechnological application, potential risk and forestalling strategy. Biotechnology Advances. 37 (1), 259-270 (2019).
- He, Y., Zhang, T., Sun, H., Zhan, H., Zhao, Y. A reporter for noninvasively monitoring gene expression and plant transformation. Horticulture Research. 7 (1), 152 (2020).
- Zheng, L., et al. An improved and efficient method of Agrobacterium syringe infiltration for transient transformation and its application in the elucidation of gene function in poplar. BMC Plant Biology. 21 (1), 54 (2021).
- Leuzinger, K., et al. Efficient agroinfiltration of plants for high-level transient expression of recombinant proteins. Journal of Visualized Experiments: JoVE. 77, 50521 (2013).
- Chincinska, I. A. Leaf infiltration in plant science: old method, new possibilities. Plant Methods. 17 (1), 83 (2021).
- Simmons, C. W., Vandergheynst, J. S., Upadhyaya, S. K. A model of agrobacterium tumefaciens vacuum infiltration into harvested leaf tissue and subsequent in planta transgene transient expression. Biotechnology and Bioengineering. 102 (3), 965-970 (2009).
- Cao, D. V., et al. Optimization of Agrobacterium -mediated transient expression of heterologous genes in spinach. Plant Biotechnology Reports. 11, 397-405 (2017).
- Xie, L., et al. Virus-induced gene silencing in the perennial woody Paeonia ostii. PeerJ. 7, ee7001 (2019).
- Prasad Babu, K., Maligeppagol, M., Asokan, R., Krishna Reddy, M. Screening of a multi-virus resistant RNAi construct in cowpea through transient vacuum infiltration method. Virusdisease. 30 (2), 269-278 (2019).
- Ekengren, S. K., Liu, Y., Schiff, M., Dinesh-Kumar, S. P., Martin, G. B. Two MARK cascades, NPR1, and TGA transcription factors play a role in Pto-mediated disease resistance in tomato. The Plant Journal: for Cell and Molecular Biology. 36 (6), 905-917 (2003).
- Deng, X., et al. Virus-induced gene silencing for Asteraceae-a reverse genetics approach for functional genomics in Gerbera hybrida. Plant Biotechnology Journal. 10 (8), 970-978 (2012).
- Wang, F., et al. Use of TRV-mediated VIGS for functional genomics research in citrus. Plant Cell, Tissue and Organ Culture. 139 (3), 609-613 (2019).
- Salazar-González, J. A., et al. In-planta transient transformation of avocado (Persea americana) by vacuum agroinfiltration of aerial plant parts. Plant Cell Tissue Organ Cult. 152, 635-646 (2023).
- Dower, W. J., Miller, J. F., Ragsdale, C. W. High efficiency transformation of E. coli by high voltage electroporation. Nucleic Acids Research. 16 (13), 6127-6145 (1988).
- GoldBio. . Electrotransformation of Agrobacterium tumefaciens Protocol. , (2018).
- Lindbo, J. A. TRBO: a high-efficiency tobacco mosaic virus RNA-based overexpression vector. Plant Physiology. 145 (4), 1232-1240 (2007).
- Rajasekaran, K., Curtis, I. S. Agrobacterium-Mediated Genetic Transformation of Cotton. Transgenic Crops of the World. , (2004).
- Llave, C., Kasschau, K. D., Carrington, J. C. Virus-encoded suppressor of posttranscriptional gene silencing targets a maintenance step in the silencing pathway. Proceedings of the National Academy of Sciences of the United States of America. 97 (24), 13401-13406 (2000).
- Fister, A. S., et al. Protocol: transient expression system for functional genomics in the tropical tree Theobroma cacao L. Plant Methods. 12, 19 (2016).
- Jung, S. -. K., et al. Agrobacterium tumefaciens mediated transient expression of plant cell wall-degrading enzymes in detached sunflower leaves. Biotechnology Progress. 30 (1), 905-915 (2014).
- Wroblewski, T., Tomczak, A., Michelmore, R. Optimization of Agrobacterium-mediated transient assays of gene expression in lettuce, tomato and Arabidopsis. Plant Biotechnology Journal. 3 (2), 259-273 (2005).
- Keith, C. V., Ramos-Sobrinho, R., Marelli, J. -. P., Brown, J. K. Construction of an infectious clone of the Badnavirus Cacao Swollen Shoot Ghana M Virus and infectivity by gene gun- and Agrobacterium-mediated inoculation. Frontiers in Agronomy. 3, 774863 (2021).
- Fister, A. S., Landherr, L., Maximova, S. N., Guiltinan, M. J. Transient expression of CRISPR/Cas9 machinery targeting TcNPR3 enhances defense response in Theobroma cacao. Frontiers in Plant Science. 9, 268 (2018).
- Grützner, R., et al. Engineering betalain biosynthesis in tomato for high level betanin production in fruits. Frontiers in Plant Science. 12, 682443 (2021).
- Saifi, S. K., Passricha, N., Tuteja, R., Kharb, P., Tuteja, N. In planta transformation: A smart way of crop improvement. Advancement in Crop Improvement Techniques. 21, 351-362 (2020).
- Huda, K. M., et al. OsACA6, a P-type IIB Ca2+ ATPase promotes salinity and drought stress tolerance in tobacco by ROS scavenging and enhancing the expression of stress-responsive genes. The Plant Journal: for Cell and Molecular Biology. 76 (6), 997-1015 (2013).
- Micheli, F., et al. Functional Genomics of Cacao. Advances in Botanical Research. 55, 119-177 (2010).
- Bailey, B. A., et al. Fungal and plant gene expression during the colonization of cacao seedlings by endophytic isolates of four Trichoderma species. Planta. 224 (6), 1449-1464 (2006).
- Motamayor, J. C., et al. Geographic and genetic population differentiation of the Amazonian chocolate tree (Theobroma cacao L). PLoS ONE. 3 (10), e3311 (2008).
Reimpresiones y Permisos
Solicitar permiso para reutilizar el texto o las figuras de este JoVE artículos
Solicitar permisoExplorar más artículos
This article has been published
Video Coming Soon
ACERCA DE JoVE
Copyright © 2025 MyJoVE Corporation. Todos los derechos reservados