Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
Vacuum-Forced Agroinfiltration for In planta Transformation of Recalcitrant Plants: Cacao as a Case Study
W tym Artykule
Podsumowanie
Here, we present the first protocol for localized vacuum infiltration for in vivo studies of the genetic transformation of large-sized plants. Using this methodology, we achieved for the first time the Agrobacterium-mediated in planta transient transformation of cacao.
Streszczenie
Transient in planta transformation is a fast and cost-effective alternative for plant genetic transformation. Most protocols for in planta transformation rely on the use of Agrobacterium-mediated transformation. However, the protocols currently in use are standardized for small-sized plants due to the physical and economic constraints of submitting large-sized plants to a vacuum treatment. This work presents an effective protocol for localized vacuum-based agroinfiltration customized for large-sized plants. To assess the efficacy of the proposed method, we tested its use in cacao plants, a tropical plant species recalcitrant to genetic transformation. Our protocol allowed applying up to 0.07 MPa vacuum, with repetitions, to a localized aerial part of cacao leaves, making it possible to force the infiltration of Agrobacterium into the intercellular spaces of attached leaves. As a result, we achieved the Agrobacterium-mediated transient in planta transformation of attached cacao leaves expressing for the RUBY reporter system. This is also the first Agrobacterium-mediated in planta transient transformation of cacao. This protocol would allow the application of the vacuum-based agroinfiltration method to other plant species with similar size constraints and open the door for the in planta characterization of genes in recalcitrant woody, large-size species.
Wprowadzenie
Plant genetic transformation methods are essential for testing the biological functions of genes and are especially useful today given the large number of uncharacterized genes predicted in the post-genomic era1. These methods can be used to obtain fully transformed lines or to express genes transiently. Stable transformation occurs when the foreign DNA the host has taken up becomes fully and irreversibly integrated into the host genome, and the genetic modifications are passed down to subsequent generations. Transient expression, known as transient transformation, occurs from the multiple copies of T-DNA transferred by Agrobacterium into the cell, which have not been integrated into the host genome, and peaks 2-4 days post infection2.
It is worth noting that transient expression assays are often sufficient for the functional characterization of genes and can offer several advantages over stable transformation. For example, transient transformation does not require tissue culture-based regeneration procedures. Another advantage is that it is compatible with in planta functional analysis of genes, existing several successful examples of protocols well standardized for model plant species, such as Arabidopsis thaliana3 and Nicotiana benthamiana4, but still limited in non-model species5.
The development of transient assays relies on the availability of efficient gene transfer methods. For this, the most popular approaches are based on Agrobacterium infiltration, which takes advantage of Agrobacterium's unique ability to transfer DNA to plant cells6. Another useful tool for these analyses is the use of reporter genes, such as green fluorescent proteins (GFP), β-glucuronidase (GUS), luciferase, or RUBY, all of which are employed to track transformation events. Among these reporter systems, RUBY is currently the easiest to visualize and relies on the conversion of tyrosine into betalains through three enzymatic step reactions. As opposed to other reporter systems, the resultant betalains can be readily observed as brightly colored pigments on transformed plant tissue without the need for sophisticated equipment or additional reactants7.
When infiltrating an Agrobacterium suspension into the intercellular space of the leaf mesophyll, the most critical step for successful agroinfection is overcoming the physical barrier imposed by the epidermal cuticle of the leaves8. While for some plants, a pressure gradient created with a needle-less syringe (syringe Agroinfiltration) is enough for an efficient agroinfiltration, as occurs in Nicotiana benthamiana9, other plant species may require a larger pressure gradient such as the one created with the help of vacuum pumps10. In vacuum-assisted processes, agroinfiltration occurs in two steps. In the first one, vacuum serves to subject the plant material to reduced pressure, forcing the release of gases from the mesophyll air spaces through stomata and wounds. Then, during a repressurization phase, the Agrobacterium suspension infiltrates the intercellular spaces via the stomata and wounds11.
Compared to syringe infiltration, vacuum infiltration allows for higher usage frequency, repeatability, and the ability to control pressure and duration at every stage of the infiltration process10. In leaves of different plant species such as spinach (Spinacia oleracea)12, peony (a woody perennial) (Paeonia ostii)13, and Cowpea (Vigna unguiculata)14, vacuum agroinfiltration protocols achieved a deeper infiltration rate than syringe infiltration. Similarly, in tomato (Lycopersicon esculentum)15, and gerbera (Gerbera hybrida)16, vacuum agroinfiltration produced stronger and more uniform gene silencing than syringe infiltration. An additional advantage of vacuum infiltration is the lower dependence on genotype, compared to syringe infiltration, which was observed recently in three citrus varieties (Fortunella obovata, Citrus limon, and C. grandis)17. However, when trying to apply vacuum agroinfiltration to plants that are too large to fit into desiccators, the size of the vacuum chambers can be a limitation, as typically occurs with tropical woody plants.
Below, we describe a protocol that overcomes the spatial limitation of vacuum chambers, testing its utility for in planta transient transformation of cacao leaves. We present the first localized vacuum infiltration method for cacao, which does not require additional equipment and even allows the use of the same laboratory desiccators used for the infiltration of the whole plant, but with a simple adaptation that allows the access of a part of the plant inside the vacuum chamber, allowing its use at different stages of plant development. To test the usefulness of the localized vacuum infiltration method proposed, we selected cacao as a proxy of a large-leaved tropical plant species that is difficult to transform. Using this localized infiltration method, we recently reported the first in planta transient expression in avocado by Agrobacterium-mediated vacuum infiltration with conditions previously optimized for detached leaves18, and here we report the first in planta transient expression in cacao.
Protokół
1. Agrobacterium tumefaciens culture
- Thaw electrocompetent cells of Agrobacterium tumefaciens strain LBA4404.
- Add 1 mL of Yeast Malt (YM; Table 1) broth to a 17 mm x 100 mm culture tube. Save this tube for later, and keep it at room temperature (RT).
- In a 1.5 mL microfuge tube, add 30 µL of the thawed Agrobacterium cells and 100-250 ng (up to 5 µL) of the DNA containing 35S:RUBY. Mix gently.
NOTE: The 35S:RUBY was a gift from Yunde Zhao. To avoid sample arcing, reduce the presence of ionic compounds as much as possible. These ionic compounds may be residual salts from the ethanol precipitation of DNA19. - At this point, place a 1 mm electroporation cuvette on ice.
- Transfer the previous suspension mix to a chilled 1 mm electroporation cuvette. Keep everything on ice. Wipe the metallic electrodes of the cuvette.
- Set the electroporator to Agr (2.2 kV, ~5 ms, 1 pulse). Place the cuvette inside the electroporation chamber.
- Press the Pulse button. Register the pulse parameters. If the sample arced, the electroporation process failed.
NOTE: It is critical to quickly transfer the cells to the YM broth right after the pulse. Delaying this transfer can dramatically reduce the transformation efficiency20. - Immediately, use the saved YM broth to transfer the cells from the cuvette to the 17 mm x 100 mm tube. Resuspend the cells gently.
- Incubate the transformed cells for 3 h at 28 °C and 250 rpm on an orbital incubator.
NOTE: This culture does not have antibiotics; be cautious about proper aseptic conditions. - Streak this culture onto selective YM agar plates21. For the transformed LBA4404-RUBY strain, ensure these plates contain rifampicin (25 µg/mL), spectinomycin (50 µg/mL), and streptomycin (50 µg/mL). Incubate this plate overnight in a 28 °C standing incubator.
NOTE: In this protocol, the vector 35S:RUBY was used, which confers bacterial resistance to spectinomycin (50 µg/mL) and functions as a visual reporter on infiltrated plant tissue. - Inoculate colonies from the overnight culture on 12.5 mL of a mixture of YM broth and Luria Bertani (LB) broth (in a 9:1 proportion, respectively), 10 mM of MES, pH 5.722. Ensure that this selective liquid medium contains the same antibiotics used in step 1.10. Refer to Table 1 to see the ingredients and concentrations for these mediums.
- When incubating Agrobacterium, leave enough aeration space for the culture, about 4 to 5 times the liquid volume. Use YM broth for Agrobacterium strain LBA4404 to avoid cell clumping23.
- Incubate the culture for 16 h at 250 rpm on a 28 °C orbital incubator.
- Scale the culture up to 10 times the initial volume with the same medium used in step 1.11.
- Incubate the culture for 16 h and 250 rpm on a 28 °C orbital incubator.
- Adjust the overnight culture to an optical density (OD600) of 0.4. Add 20 µM of acetosyringone (AS).
- Incubate at 250 rpm on a 28 °C orbital incubator until OD600 reaches about 1.0.
- Centrifuge the cells at 4 500 x g for 10 min at 20 °C.
- Resuspend the pellet with suspension solution (10 mM MES, 10 mM MgCl2, pH 5.7), adjusting OD600 to 0.6. Add 200 µM AS24.
NOTE: Pre-incubate the suspension solution at 28 °C. If the suspension solution is cold when added, the cells will precipitate. - Leave the bacterial suspension for 2-24 h in dark conditions and 25 °C. Agitation is not required22.
2. Plant selection
- Choose a plant with a branch with leaves in the optimal stage for agroinfiltration.
NOTE: The plant may be full-grown or a mature tree. For cacao, young leaves of C stage are recommended. These leaves are bronze to light green colored; they are not fully expanded nor as rigid as stage D leaves25(Figure 1).- As a control, simultaneously perform agroinfiltration on other plants (e.g., Nicotiana tabacum) with a high agroinfiltration efficiency that is reported for the strain and vector used.
NOTE: If no positive results are obtained in this control, it is possible that the negative results are due to the strain or the vector used.
- As a control, simultaneously perform agroinfiltration on other plants (e.g., Nicotiana tabacum) with a high agroinfiltration efficiency that is reported for the strain and vector used.
3. Vacuum chamber setup
- As a vacuum chamber, use a desiccator that has a vacuum gauge to measure the vacuum pressure inside.
- Add 250 µM of Jasmonic acid (JA)18,26 to the Agrobacterium suspension from step 1.19.
- Transfer the Agrobacterium culture to a wide-mouth beaker to submerge the selected branch and leaves. Then, place the beaker with Agrobacterium culture inside the desiccator.
- Place the branch between the desiccator and its lid. Make sure to submerge the desired leaves inside the Agrobacterium culture. Next, use a jump ring, which is a round ring with a cutout that allows the plant branch to enter the desiccator. The Jump Ring also acts as a spacer between the bottom and top of the Desiccator.
- Ensure that the gasket is structurally stable enough to avoid being squished with the lid, flexible enough to bend and adjust to the circumference of the desiccator, and not made of a porous material.
NOTE: This study used a stranded metal wire consisting of several smaller wires twisted together, coated with a nonporous plastic material resembling a gasket (Figure 2). - To fix the branch onto the desiccator, use silicone impression material. Ensure that the material is sticky, nonporous, chemically inert to the desiccator and the plant, and easy to apply and fill small gaps between the branch, the gasket, and the desiccator.
- Once the silicone impression material polymerizes (this takes about 1 min) and fixes up the branch in place, close the desiccator. Make sure not to leave any gaps.
- Connect the desiccator to the vacuum pump (Figure 3).
4. Vacuum infiltration
- Start the vacuum pump until it gets to -0.07 MPa.
- Once it reaches this pressure, close the pressure valve and turn off the vacuum pump. Maintain this pressure for 5 min.
- Open the pressure valve to restore the chamber pressure.
NOTE: This is a critical step. Restore the chamber pressure gradually and steadily. It may take up to 3 min to fully repressurize the desiccator. Extended re-pressurization increases the number of bacteria infiltrated inside the tissue10. - Repeat this process two more times.
- Take the branch off the cell suspension and the desiccator.
- Clean the infiltrated leaves with distilled water.
5. Incubation of infiltrated leaves
- Let the infiltrated leaves stay in dark conditions at 25 °C for 48 h.
- Then, expose the infiltrated tissue to a 16/8-h light/dark photoperiod.
- Evaluate the transient leaf transformation 3-7 days post-infection (DPI).

Figure 1: Cacao leaves developmental stages. (A-E) Developmental stages25. Please click here to view a larger version of this figure.
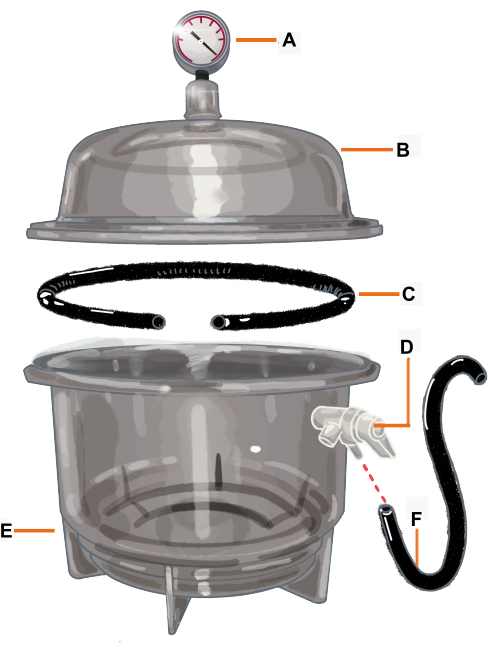
Figure 2: Vacuum chamber configuration and its components. The vacuum chamber is a desiccator connected to a vacuum gauge. The gasket/ O-ring is cut so it has an opening where the branch will be placed. (A) Vacuum gauge, (B) Lid, (C) Gasket/ O-ring, (D) Pressure valve, (E) Desiccator, (F) Hose. Please click here to view a larger version of this figure.

Figure 3: In planta vacuum agroinfiltration system. To avoid vacuum losses during the infiltration process, it is critical to secure the branch to the desiccator and the gasket/O-ring with silicone impression material. (A) Cacao plant, (B) Vacuum chamber, (C) Silicone impression material, (D) Leaves submerged on Agrobacterium suspension, (E) Vacuum pump. Please click here to view a larger version of this figure.
Wyniki
This protocol presents an effective agroinfiltration method for large-sized woody plants. With this protocol, we were able to achieve a vacuum pressure of -0.07 MPa, resulting in the effective, localized infiltration of cacao leaves. In Figure 4, we observe the infiltration system setting up process, and in Figure 5, the final configuration.
Dyskusje
In this work, we presented an efficient, low-cost agroinfiltration protocol for the in planta transient transformation of woody plants, using cacao plants as an example. Given the well-known constraint that the cuticle of leaves represents for the transformation of plant tissues, we concentrated on developing a strategy to facilitate agroinfiltration by vacuum in woody plants, which are usually recalcitrant to this procedure.
The achieved vacuum pressure inside the vacuum chamber was ...
Ujawnienia
Authors have no conflict of interest to declare.
Podziękowania
We thank Lic. Jesús Fuentes González and Néstor Iván Robles Olivares for their assistance in filming the video footage. We acknowledge the generous gifts by Dr. Antonia Gutierrez Mora of CIATEJ (Theobroma cacao plants). We also thank CIATEJ and Laboratorio Nacional PlanTECC, México, for facility support. H.E.H.D. (CVU: 1135375) conducted master studies with funding from the Consejo Nacional de Humanidades, Ciencia y Tecnología, México (CONAHCYT). R.U.L. acknowledges support from Consejo Estatal de Ciencia y Tecnología de Jalisco (COECYTJAL), and Secretaría de Innovación Ciencia y Tecnología (SICYT), Jalisco, México (Grant 7270-2018).
Materiały
| Name | Company | Catalog Number | Comments |
| 35S:RUBY plasmid | Addgene | 160908 | http://n2t.net/addgene:160908 ; RRID:Addgene_160908 |
| 1 mm electroporation cuvette | Thermo Fisher Scientific | FB101 | Fisherbrand Electroporation Cuvettes Plus |
| Desiccator | Bel-Art SP SCIENCEWARWE | F42400-2121 | |
| Freeze dryer | LABCONCO | 700402040 | |
| K2HPO4 | Sigma Aldrich | P8281-500G | For YM medium add 0.38 g/L |
| LBA4404 ElectroCompetent Agrobacterium | Intact Genomics USA | 1285-12 | https://intactgenomics.com/product/lba4404-electrocompetent-agrobacterium/ |
| Mannitol | Sigma Aldrich | 63560-250G-F | For YM medium add 10 g/L |
| MES | Sigma Aldrich | PHG0003 | (For LB, YM and resuspension medium) add 1.95 g/L (10mM) |
| MgCl2 | Sigma Aldrich | M8266 | For resuspension medium add 0.952 g/L (10 mM) |
| MgSO4·7H20 | Sigma Aldrich | 63138-1KG | For YM medium add 0.204 g/L |
| MicroPulser Electroporation Apparatus | Biorad | 165-2100 | |
| NaCl | Karal | 60552 | For LB medium add 5 g/L; For YM medium add 0.1 g/L |
| NanoDrop One Microvolume UV-Vis Spectrophotometer | Thermo Fisher Scientific | 13-400-518 | |
| President Silicone Impression material | COLTENE | 60019938 | |
| Rifampicin | Gold-Bio | R-120-1 | (100 mg/mL) |
| Silicone Impression material gun | Andent | TBT06 | |
| Spectinomycin | Gold-Bio | S-140-SL10 | (100 mg/mL) |
| Streptomycin | Gold-Bio | S-150-SL10 | (100 mg/mL) |
| Tryptone enzymatic digest from casein | Sigma Aldrich | 95039-1KG-F | For LB medium add 10 g/L |
| Yeast extract | MCD LAB | 9031 | For LB medium add 5 g/L; For YM medium add 0.4 g/L |
Odniesienia
- Yang, J., Jia, M., Guo, J., Huang, L. Q. Functional Genome of Medicinal Plants. Molecular Pharmacognosy. , (2019).
- Janssen, B. J., Gardner, R. C. Localized transient expression of GUS in leaf discs following cocultivation with Agrobacterium. Plant Molecular Biology. 14 (1), 61-72 (1990).
- Wang, X., et al. Identification and functional analysis of in vivo phosphorylation sites of the Arabidopsis BRASSINOSTEROID-INSENSITIVE1 receptor kinase. Plant Cell. 17 (6), 1685-1703 (2005).
- Pan, Z., et al. In vivo assembly of the sorgoleone biosynthetic pathway and its impact on agroinfiltrated leaves of Nicotiana benthamiana. The New Phytologist. 230 (2), 683-697 (2021).
- Manavella, P. A., Chan, R. L. Transient transformation of sunflower leaf discs via an agrobacterium-mediated method: Applications for gene expression and silencing studies. Nature Protocols. 4 (11), 1699-1707 (2009).
- Guo, M., Ye, J., Gao, D., Xu, N., Yang, J. Agrobacterium-mediated horizontal gene transfer: Mechanism, biotechnological application, potential risk and forestalling strategy. Biotechnology Advances. 37 (1), 259-270 (2019).
- He, Y., Zhang, T., Sun, H., Zhan, H., Zhao, Y. A reporter for noninvasively monitoring gene expression and plant transformation. Horticulture Research. 7 (1), 152 (2020).
- Zheng, L., et al. An improved and efficient method of Agrobacterium syringe infiltration for transient transformation and its application in the elucidation of gene function in poplar. BMC Plant Biology. 21 (1), 54 (2021).
- Leuzinger, K., et al. Efficient agroinfiltration of plants for high-level transient expression of recombinant proteins. Journal of Visualized Experiments: JoVE. 77, 50521 (2013).
- Chincinska, I. A. Leaf infiltration in plant science: old method, new possibilities. Plant Methods. 17 (1), 83 (2021).
- Simmons, C. W., Vandergheynst, J. S., Upadhyaya, S. K. A model of agrobacterium tumefaciens vacuum infiltration into harvested leaf tissue and subsequent in planta transgene transient expression. Biotechnology and Bioengineering. 102 (3), 965-970 (2009).
- Cao, D. V., et al. Optimization of Agrobacterium -mediated transient expression of heterologous genes in spinach. Plant Biotechnology Reports. 11, 397-405 (2017).
- Xie, L., et al. Virus-induced gene silencing in the perennial woody Paeonia ostii. PeerJ. 7, ee7001 (2019).
- Prasad Babu, K., Maligeppagol, M., Asokan, R., Krishna Reddy, M. Screening of a multi-virus resistant RNAi construct in cowpea through transient vacuum infiltration method. Virusdisease. 30 (2), 269-278 (2019).
- Ekengren, S. K., Liu, Y., Schiff, M., Dinesh-Kumar, S. P., Martin, G. B. Two MARK cascades, NPR1, and TGA transcription factors play a role in Pto-mediated disease resistance in tomato. The Plant Journal: for Cell and Molecular Biology. 36 (6), 905-917 (2003).
- Deng, X., et al. Virus-induced gene silencing for Asteraceae-a reverse genetics approach for functional genomics in Gerbera hybrida. Plant Biotechnology Journal. 10 (8), 970-978 (2012).
- Wang, F., et al. Use of TRV-mediated VIGS for functional genomics research in citrus. Plant Cell, Tissue and Organ Culture. 139 (3), 609-613 (2019).
- Salazar-González, J. A., et al. In-planta transient transformation of avocado (Persea americana) by vacuum agroinfiltration of aerial plant parts. Plant Cell Tissue Organ Cult. 152, 635-646 (2023).
- Dower, W. J., Miller, J. F., Ragsdale, C. W. High efficiency transformation of E. coli by high voltage electroporation. Nucleic Acids Research. 16 (13), 6127-6145 (1988).
- GoldBio. . Electrotransformation of Agrobacterium tumefaciens Protocol. , (2018).
- Lindbo, J. A. TRBO: a high-efficiency tobacco mosaic virus RNA-based overexpression vector. Plant Physiology. 145 (4), 1232-1240 (2007).
- Rajasekaran, K., Curtis, I. S. Agrobacterium-Mediated Genetic Transformation of Cotton. Transgenic Crops of the World. , (2004).
- Llave, C., Kasschau, K. D., Carrington, J. C. Virus-encoded suppressor of posttranscriptional gene silencing targets a maintenance step in the silencing pathway. Proceedings of the National Academy of Sciences of the United States of America. 97 (24), 13401-13406 (2000).
- Fister, A. S., et al. Protocol: transient expression system for functional genomics in the tropical tree Theobroma cacao L. Plant Methods. 12, 19 (2016).
- Jung, S. -. K., et al. Agrobacterium tumefaciens mediated transient expression of plant cell wall-degrading enzymes in detached sunflower leaves. Biotechnology Progress. 30 (1), 905-915 (2014).
- Wroblewski, T., Tomczak, A., Michelmore, R. Optimization of Agrobacterium-mediated transient assays of gene expression in lettuce, tomato and Arabidopsis. Plant Biotechnology Journal. 3 (2), 259-273 (2005).
- Keith, C. V., Ramos-Sobrinho, R., Marelli, J. -. P., Brown, J. K. Construction of an infectious clone of the Badnavirus Cacao Swollen Shoot Ghana M Virus and infectivity by gene gun- and Agrobacterium-mediated inoculation. Frontiers in Agronomy. 3, 774863 (2021).
- Fister, A. S., Landherr, L., Maximova, S. N., Guiltinan, M. J. Transient expression of CRISPR/Cas9 machinery targeting TcNPR3 enhances defense response in Theobroma cacao. Frontiers in Plant Science. 9, 268 (2018).
- Grützner, R., et al. Engineering betalain biosynthesis in tomato for high level betanin production in fruits. Frontiers in Plant Science. 12, 682443 (2021).
- Saifi, S. K., Passricha, N., Tuteja, R., Kharb, P., Tuteja, N. In planta transformation: A smart way of crop improvement. Advancement in Crop Improvement Techniques. 21, 351-362 (2020).
- Huda, K. M., et al. OsACA6, a P-type IIB Ca2+ ATPase promotes salinity and drought stress tolerance in tobacco by ROS scavenging and enhancing the expression of stress-responsive genes. The Plant Journal: for Cell and Molecular Biology. 76 (6), 997-1015 (2013).
- Micheli, F., et al. Functional Genomics of Cacao. Advances in Botanical Research. 55, 119-177 (2010).
- Bailey, B. A., et al. Fungal and plant gene expression during the colonization of cacao seedlings by endophytic isolates of four Trichoderma species. Planta. 224 (6), 1449-1464 (2006).
- Motamayor, J. C., et al. Geographic and genetic population differentiation of the Amazonian chocolate tree (Theobroma cacao L). PLoS ONE. 3 (10), e3311 (2008).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaPrzeglądaj więcej artyków
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone