A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Limbal Approach-Subretinal Injection of Viral Vectors for Gene Therapy in Mice Retinal Pigment Epithelium
In This Article
Summary
Subretinal injection is a surgical technique for effective gene delivery to retinal pigment epithelium in the mouse eye. Here we describe an easy and replicable method for subretinal injection of viral vectors to retinal pigment epithelium in experimental mice.
Abstract
The eye is a small and enclosed organ which makes it an ideal target for gene therapy. Recently various strategies have been applied to gene therapy in retinopathies using non-viral and viral gene delivery to the retina and retinal pigment epithelium (RPE). Subretinal injection is the best approach to deliver viral vectors directly to RPE cells. Before the clinical trial of a gene therapy, it is inevitable to validate the efficacy of the therapy in animal models of various retinopathies. Thus, subretinal injection in mice becomes a fundamental technique for an ocular gene therapy. In this protocol, we provide the easy and replicable technique for subretinal injection of viral vectors to experimental mice. This technique is modified from the intravitreal injection, which is widely used technique in ophthalmology clinics. The representative results of RPE/choroid/scleral complex flat-mount will help to understand the efficacy of this technique and adjust the volume and titer of viral vectors for the extent of gene transduction.
Introduction
In ophthalmology, gene therapy has emerged as the treatment modality in monogenic inherited retinopathies. There are inherited retinopathies associated with genes in retinal pigment epithelium (RPE) including Leber congenital amurosis1,2, retinitis pigmentosa3, and choroideremia4. The research field of gene therapy is expanding in both preclinical studies and clinical trials using viral vectors such as adeno-associated virus (AAV), lentivirus (LV) and adenovirus (Ad)5. Different viral vectors have different tropism in the retina. For a safe and effective gene therapy, viral vectors should be carefully selected according to target cells and target genes.
The route of gene delivery is also important for effective gene delivery to target cells, thus, it should be carefully chosen as well. The two most common methods for intraocular delivery of viral vectors are subretinal injection and intravitreal injection6. The latter, intravitreal injection, has been widely used for drug delivery to treat choroidal neovascularization in wet age-related macular degeneration (AMD) and macular edema in diabetic retinopathy7. Intravitreal route provides exposure of viral vectors to vitreous and inner retina, but the diffusion of the vectors to outer retina is limited. On the other hand, the subretinal route provides direct delivery of viral vectors to the potential space between retina and RPE, inducing a localized bleb. Therefore, subretinal injection is currently considered a more efficient route for targeting photoreceptor cells and RPE. In terms of surgical approach, pars plana is chosen as a safe area for intravitreal injection to avoid retinal damage in human patients. By simply modifying this approach to mice, we could inject viral vectors subretinally or intravireally via limbal approach.
In this video article, we demonstrate an easy and convenient method of subretinal injection of viral vectors into mice RPE. After single puncture at posterior to limbus with a 30 G 1/2 needle, a 33 G blunt needle equipped microliter syringe is inserted into the subretinal space via the limbal puncture site. The viral vectors of 1.5 - 2 µl volume are injected to the potential space between retina and RPE inducing subretinal blebs. This procedure can be performed under direct visualization using surgical microscope. Repeated practice will guarantee replicable results even without direct visualization of the bleb formation. This will help the researchers to perform accurate and timesaving experiments for subretinal gene delivery in mice RPE.
Protocol
All experiments on animals were performed in accordance with the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research, and the guidelines and regulations set forth by the Seoul National University Institutional Animal Care and Use Committee and Seoul National University Hospital Biosafety Committee.
1. Preparing Injection Kit and Viral Vectors
- Prepare the microliter syringe equipped with a 33 G blunt needle sterilized using ethylene oxide gas. Dilute the viral vectors in PBS for the adequate titer in the micro tube (i.e., 1 x 106 TU/µl). Flush the syringe several times with viral vectors to remove any dead space in the syringe.
2. Subretinal Injection of Viral Vectors
- Anesthetize the adult mice (i.e., 6 - 8 weeks old) with an intraperitoneal injection of the mixture of tiletamine and zolazepam (1:1, 2.25 mg/kg body weight) and xylazine hydrochloride (0.7 mg/kg body weight) or an alternative suitable anesthesia regime.
- Dilate pupils with an eye drop of phenylephrine 0.5% and tropicamide 0.5%.
- Prepare the microliter syringe by loading with 1.5 - 2 µl of viral vectors.
- Open the eyelid and protrude the eye to expose the equator for convenient injection and focus on under the operating microscope. Maintain the protruded eye position until finishing the injection, or displacement of the needle can occur during the injection. To hold the eyeball firmly, place the fingers outside the orbital rim.
- Apply a drop of ophthalmic viscoelastic solution to the corneal surface.
- Place a small round cover slide on the top of the cornea to visualize the retina.
- Puncture a small hole at slight posterior to the limbus using a sterile 30 G 1/2 needle for the further subretinal injection. Make the hole inferior for the right eye, and superior for the left eye for the convenience.
NOTE: If the hole is made at temporal or nasal, it’s hard to be covered by the eyelid after the injection. Initial puncture should be made slightly posterior to the limbus to avoid the limbal vessels which run along the limbus and can be easily recognized. Be careful to avoid hitting the lens with needle while making the initial puncture. Do not insert the entire bevel of the needle to avoid lens puncture. - Place the 33 G blunt needle of microliter syringe through the pre-punctured hole and approach the needle into the subretinal space until the point when mild resistance is felt.
NOTE: For the subretinal injection, the best approach angle of the needle is about 45 degrees against iris plane, and the blunt needle should be pushed posteriorly toward peripapillary area. Dotted square indicated the suggested needle pathway across the vitreous cavity for the subretinal injection in Figure 1.
NOTE: There will be no resistance felt when piercing (or passing) the retinal layers as they are very soft. Thus, the first feeling of mild resistance indicates that the needle has already touched the RPE layer and the insertion of the needle should be stopped. Be careful not to penetrate scleral tissue with excessive pressure because the needle should be placed in the potential subretinal space. If the needle punctures the scleral tissue by excessive power, it enters into the extraocular orbital spaces without resistance. (This step is critical)
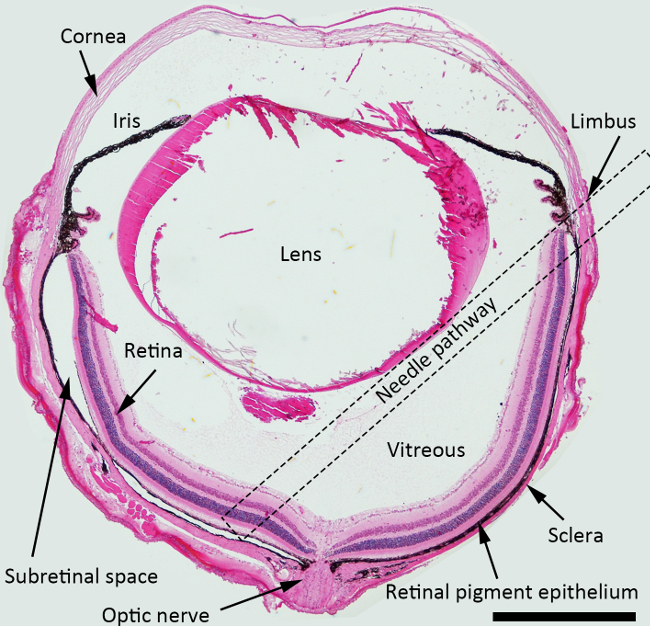
Figure 1. Schematic Diagram of the Subretinal Injection. H&E stained cross section of the mouse eye depicting the structures with a needle pathway for the subretinal injection marked (a dotted square). Magnification:40X, Scale bar: 500 µm. Please click here to view a larger version of this figure.
- Inject the viral vectors (i.e., 1 x 106 TU/µl) gently into the subretinal space without tremor to avoid unwanted tissue damage and withdraw the needle gently. Hold the eyeball firmly during the injection as described in 2.4.
- Observe the formation of subretinal bleb after the injection under operating microscope to make sure there is no retinal bleeding.
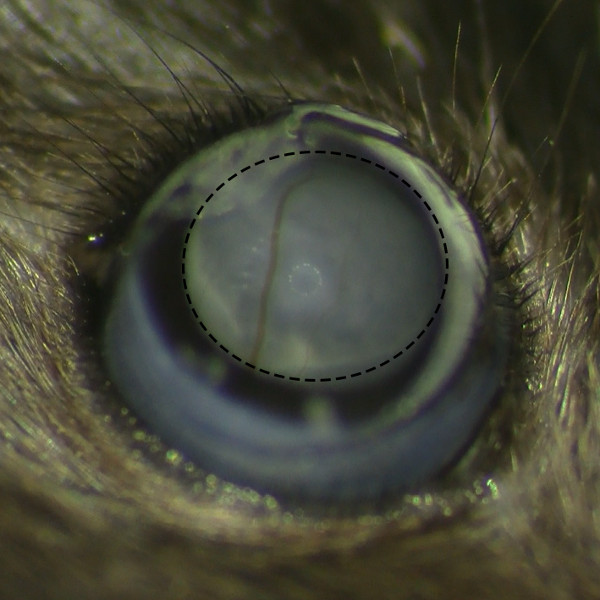
Figure 2. Subretinal Belb Formation without Retinal Hemorrhage. Microscopic view of subretinal belb after the subretinal injection under operating microscope shows successful bleb formation without retinal hemorrhage. Please click here to view a larger version of this figure.
- Gently close the eyelid to cover the injection site for self-sealing. Return the mice to home cage and keep alive until the evaluation.
NOTE: After the researchers get used to the procedures, they can get the repeatable results with rapid procedures by skipping these steps (2.5, 6, and 10)
3. Evaluation of the Efficacy of Gene Delivery in Retinal Pigment Epithelium
- Sacrifice the mice in the CO2 chamber. Enucleate the eyes using scissors.
- Fix the eyes in the 4% paraformaldehyde solution at 4 °C for a minimum 1 hr to few days.
- Grab the cornea with fine forceps and puncture the cornea with micro-scissors. Remove the cornea after scissoring along the limbus. Remove the lens with forceps.
- Trim the ciliary body with micro-scissors allowing retinal detachment. Gently pull the whole retina from the RPE/choroid/sclera complex. Cut the RPE/choroid/scleral complex radially from the periphery to the center near optic nerve several times to make 4 to 8 leaves for flat mounting.
- Flip the RPE/choroid/scleral complex to make RPE side down, and trim all the remaining tissues at the scleral side including muscles, conjunctiva and optic nerve. This step is important to make a flat RPE/choroid/scleral complex because any remaining tissue makes the complex uneven.
- Incubate the complex with primary and secondary antibody for your specific research purposes (optional, see the references for detailed methods). Flat-mount the RPE/choroid/scleral complexes on the glass slide and absorb the PBS with an absorbent sponge.
- Add 30 µl of mounting solution and place the cover slide. Observe the sample for the efficacy of vector delivery under fluorescein microscope. Keep the samples in the refrigerator for further observation.
Results
To evaluate the efficacy of the subretinal injection on viral gene transduction by this protocol, we used commercially available LV vectors with CMV promoter expressing both GFP and RFP for the indicator. Eyes were enucleated after the appropriate time period according to the research purpose. For the representative results, eyes were enucleated 10 weeks and 20 weeks after subretinal injection. After complete removal of the retina using the method described above, the flat mount of RPE/choroid/sclera complex was evaluate...
Discussion
In this video article, we described the limbal-approach subretinal injection technique in detail with representative results of RPE/choroid/scleral flat-mount. This is an easy and convenient technique for subretinal injection of viral vectors into RPE. Direct visualization of bleb formation during the injection is an important step for accurate delivery for the beginners. There are some subretinal injection techniques introduced in Journal of Visualized Experiments8-10. Subretinal space is the potential space ...
Disclosures
The authors have nothing to disclose.
Acknowledgements
This study was supported by the Seoul National University Research Grant (800-20140542), the Pioneer Research Program of NRF/MEST (2012-0009544), and the Bio-Signal Analysis Technology Innovation Program of NRF/MEST (2009-0090895), and the Grant of NRF/MEST (2015M3A9E6028949).
Materials
| Name | Company | Catalog Number | Comments |
| TWEEZERS DUMONT #5 11cm DUMOSTAR 0.1 x 0.06 mm TIPS | WPI | 500233 | |
| VANNAS Scissors S/S, 105mm | WPI | 555583S | |
| 33G Blunt needle | WPI | NF33BL-2 | |
| NanoFil Syringe, 10 microliter | WPI | NANOFIL | |
| RPE-KIT | WPI | RPE-KIT | For easy one hand injection |
| 30Gx1/2 (0.3mmx 13mm) BD PrecisionGlideTM Needle | BD | 305107 | Initial puncture for subretinal injection |
| Microscope Cover Glasses (No. 1 3 mm diameter) | Warner Instruments | 64-0720 (CS-3R) | |
| Leica operating microscope | Leica | LM M80 | |
| Fluoresecein microscope | Nikon | Eclipse 80i | |
| Lentivirus | Thermo scientific | TMO.LV-Ctr | Used to dilute vectors |
| PBS | Gibco | 10010-015 | Used to dilute vectors |
| Troperin (Phenylephrin 0.5%-Tropicamide 0.5%) | Hanmi | For dilation | |
| Proparacaine Hydrochloride Ophthalmic Solution USP, 0.5% (Sterile) | Bausch&Lomb | For topical anesthesia | |
| Healon GV OVD | Abbott Medical Optics Inc. | ||
| Zoletil 50 (tiletamine hypochloride and zolazepam hypochloride) | Virbac | For general anesthesia | |
| Rompun® injection (Xylazine HCl) | Bayer | For general anesthesia |
References
- Maguire, A. M., et al. Safety and efficacy of gene transfer for Leber's congenital amaurosis. N Engl J Med. 358 (21), 2240-2248 (2008).
- Jacobson, S. G., et al. Gene therapy for leber congenital amaurosis caused by RPE65 mutations: safety and efficacy in 15 children and adults followed up to 3 years. Arch Ophthalmol. 130 (1), 9-24 (2012).
- Conlon, T. J., et al. Preclinical potency and safety studies of an AAV2-mediated gene therapy vector for the treatment of MERTK associated retinitis pigmentosa. Hum Gene Ther Clin Dev. 24 (1), 23-28 (2013).
- MacLaren, R. E., et al. Retinal gene therapy in patients with choroideremia: initial findings from a phase 1/2 clinical trial. Lancet. 383 (9923), 1129-1137 (2014).
- Trapani, I., Puppo, A., Auricchio, A. Vector platforms for gene therapy of inherited retinopathies. Prog Retin Eye Res. 43, 108-128 (2014).
- Liang, F. Q., Anand, V., Maguire, A. M., Bennett, J. Intraocular delivery of recombinant virus. Methods Mol Med. 47, 125-139 (2001).
- Peyman, G. A., Lad, E. M., Moshfeghi, D. M. Intravitreal injection of therapeutic agents. Retina. 29 (7), 875-912 (2009).
- Matsumoto, H., Miller, J. W., Vavvas, D. G. Retinal detachment model in rodents by subretinal injection of sodium hyaluronate. J Vis Exp. (79), (2013).
- Wert, K. J., Skeie, J. M., Davis, R. J., Tsang, S. H., Mahajan, V. B. Subretinal injection of gene therapy vectors and stem cells in the perinatal mouse eye. J Vis Exp. (69), (2012).
- Eberle, D., Santos-Ferreira, T., Grahl, S., Ader, M. Subretinal transplantation of MACS purified photoreceptor precursor cells into the adult mouse retina. J Vis Exp. (84), e50932 (2014).
- Sahel, J. A., Roska, B. Gene therapy for blindness. Annu Rev Neurosci. 36, 467-488 (2013).
- Allocca, M., et al. Novel adeno-associated virus serotypes efficiently transduce murine photoreceptors. J Virol. 81 (20), 11372-11380 (2007).
- Takahashi, K., et al. Sustained transduction of ocular cells with a bovine immunodeficiency viral vector. Hum Gene Ther. 13 (11), 1305-1316 (2002).
- Rolling, F., et al. Gene therapeutic prospects in early onset of severe retinal dystrophy: restoration of vision in RPE65 Briard dogs using an AAV serotype 4 vector that specifically targets the retinal pigmented epithelium. Bull Mem Acad R Med Belg. 161 (10-12), 497-508 (2006).
- Le Meur, G., et al. Restoration of vision in RPE65-deficient Briard dogs using an AAV serotype 4 vector that specifically targets the retinal pigmented epithelium. Gene Ther. 14 (4), 292-303 (2007).
- Alexander, J. J., Hauswirth, W. W. Adeno-associated viral vectors and the retina. Adv Exp Med Biol. 613, 121-128 (2008).
- Puche, N., et al. Genetic and environmental factors associated with reticular pseudodrusen in age-related macular degeneration. Retina. 33 (5), 998-1004 (2013).
- Campochiaro, P. A. Gene transfer for neovascular age-related macular degeneration. Hum Gene Ther. 22 (5), 523-529 (2011).
- Park, S. W., et al. Intracellular amyloid beta alters the tight junction of retinal pigment epithelium in 5XFAD mice. Neurobiol Aging. 35 (9), 2013-2020 (2014).
- Shin, B., et al. Intracellular cleavage of amyloid beta by a viral protease NIa prevents amyloid beta-mediated cytotoxicity. PLoS One. 9 (6), e98650 (2014).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved