A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Using Modified Synthetic Oligonucleotides to Assay Nucleic Acid-Metabolizing Enzymes
In This Article
Summary
Here, a protocol for assaying nucleic acid metabolizing enzymes is presented, using examples of ligase, nuclease, and polymerase enzymes. The assay utilizes fluorescently labeled and unlabeled oligonucleotides that can be combined to form duplexes mimicking RNA and/or DNA damages or pathway intermediates, allowing for the characterization of enzyme behavior.
Abstract
The availability of a range of modified synthetic oligonucleotides from commercial vendors has allowed the development of sophisticated assays to characterize diverse properties of nucleic acid metabolizing enzymes that can be run in any standard molecular biology lab. The use of fluorescent labels has made these methods accessible to researchers with standard PAGE electrophoresis equipment and a fluorescent-enabled imager, without using radioactive materials or requiring a lab designed for the storage and preparation of radioactive materials, i.e., a Hot Lab. The optional addition of standard modifications such as phosphorylation can simplify assay setup, while the specific incorporation of modified nucleotides that mimic DNA damages or intermediates can be used to probe specific aspects of enzyme behavior. Here, the design and execution of assays to interrogate several aspects of DNA processing by enzymes using commercially available synthetic oligonucleotides are demonstrated. These include the ability of ligases to join or nucleases to degrade different DNA and RNA hybrid structures, differential cofactor usage by the DNA ligase, and evaluation of the DNA-binding capacity of enzymes. Factors to consider when designing synthetic nucleotide substrates are discussed, and a basic set of oligonucleotides that can be used for a range of nucleic acid ligase, polymerase, and nuclease enzyme assays are provided.
Introduction
All life forms require nucleic acid processing enzymes to carry out fundamental biological processes, including replication, transcription, and DNA repair. Key enzymatic functionalities for these pathways are polymerases, which generate copies of RNA/DNA molecules, ligases which join polynucleotide substrates, nucleases that degrade them, and helicases and topoisomerases, which melt nucleic acid duplexes or change their topology1,2,3,4,5,6,7,8,9,10. In addition, many of these enzymes provide essential molecular tools for applications such as cloning, diagnostics, and high-throughput sequencing11,12,13,14,15.
The functional characteristics, kinetics, and substrate specificities of these enzymes can be determined using labeled DNA/RNA substrates produced by annealing oligonucleotides. Tracking substrates and products has traditionally been achieved by introducing a radioactive label (32P) at either the 5' strand end, which can then be detected by photographic film or with a phosphor imaging system16,17. While radiolabeled substrates offer the benefit of increased experimental sensitivity and do not alter the chemical properties of a nucleotide, the potential health hazards from working with radioisotopes have encouraged the development of non-radioactive nucleic acid labeling to provide a safer alternative for DNA and RNA detection18,19,20. Among these, fluorescence detection, including direct fluorescence detection, time-resolved fluorescence, and energy transfer/fluorescence quenching assays stand out as the most versatile21,22,23,24. The extensive array of fluorophores enables different designs of DNA/RNA substrates featuring unique reporters on each oligonucleotide25. Additionally, the stability of fluorophores, when compared to radioisotopes, allows users to produce and preserve significant quantities of fluorescently labeled DNA substrates19. These fluorophore-labeled substrates can be incubated with the protein of interest, along with different combinations of metal and nucleotide cofactors, to analyze binding and or enzyme activity. Visualization of binding or activity can be observed using various fluorophore dye channels with a gel imaging system. As only the fluorescently labeled oligonucleotides will be visible using this technique, any increase or decrease in the size of the labeled oligonucleotide will be easy to follow. Gels can also be stained afterward, with nucleic acid staining dyes to visualize all DNA bands present on the gel.
Poly-nucleic acid ligases are enzymes that join fragments of DNA/RNA, catalyzing the sealing of breaks by the formation of a phosphodiester bond between 5' phosphorylated DNA termini and the 3' OH of DNA. They can be divided into two groups according to their nucleotide substrate requirement. The highly conserved NAD-dependent ligases are found in all bacteria26 while the structurally diverse ATP-dependent enzymes can be identified through all domains of life8,27. DNA ligases play an important role in Okazaki fragment processing during replication as well as being involved in various DNA repair pathways, such as nucleotide and base excision repair, through the sealing of spontaneous nicks and nicks that are left after repair8,10. Different DNA ligases exhibit varying capacities to join different conformations of DNA breaks, including nicks in a duplex, double-stranded breaks, mismatches, and gaps, as well as RNA and DNA hybrids28,29,30. A diverse range of ligatable substrates can be assembled by annealing oligonucleotides with a 5' phosphate to generate juxtaposed 5' and 3' termini in a nucleic acid duplex31,32,33. The most common method of analysis is resolution by urea PAGE in an end-point assay format; however, recent innovations have included the use of capillary gel electrophoresis, which allows high throughput34, mass-spectrometric profiling35, as well as a homogenous molecular beacon assay, which allows time-resolved monitoring36.
The first step in a ligation reaction is the adenylation of the ligase enzyme by adenosine triphosphate (ATP) or Nicotinamide adenine dinucleotide (NAD), resulting in a covalent enzyme intermediate. The second step in the reaction is adenylation of the nucleic acid substrate on the 5' end of the nick site, which is followed by ligation of the nucleic acid nick strands. Many ligase enzymes that are recombinantly expressed in E. coli are purified in the adenylated form and, therefore, are able to successfully ligate nucleic acids without the addition of a nucleotide cofactor. This makes it difficult to determine what particular type of nucleotide cofactor they require for the ligation of nucleic acids. In addition to describing assays to evaluate DNA ligase activity, a method to reliably determine the cofactor usage by de-adenylating the enzyme using unlabeled substrates is also presented.
Nucleases are a large and diverse group of DNA/RNA modifying enzymes and catalytic RNAs that cleave the phosphodiester bonds between nucleic acids37. Nuclease enzyme functionalities are required in DNA replication, repair, and RNA processing and can be classified by their sugar specificity for DNA, RNA, or both. Endonucleases hydrolyze the phosphodiester bonds within a DNA/RNA strand, while exonucleases hydrolyze DNA/RNA strands one nucleotide at a time from the 3' or 5' end and may do so from either the 3' to 5' or the 5' to 3' end of the DNA38.
While many nuclease proteins are non-specific and may be involved in multiple processes, others are highly specific for a particular sequence or DNA damage6,39,40. Sequence-specific nucleases are used in a wide range of biotechnological applications, such as cloning, mutagenesis, and genome editing. Popular nucleases for these applications are restriction nucleases41, zinc-finger nucleases42, transcriptional activator-like effector nucleases, and most recently, the RNA-guided engineered CRISPR nucleases43. Damage-specific nucleases have recently been identified, such as the EndoMS nuclease, which has specificity for mismatches in the DNA through its mismatch-specific RecB-like nuclease domain5,44. Nuclease activity assays, historically, have been done as discontinuous assays with radiolabeled substrates; however, in addition to their other drawbacks, these do not allow the identification of the site that is cut by a nuclease protein, which is possible when using fluorescently labeled substrates45,46. More recently, continuous nuclease assays have been developed which work by using different DNA dyes that interact with DNA in different states; for example, emitting a higher fluorescent signal when interacting with dsDNA than in its unbound state, or binding specifically to short RNAs47. Other continuous nuclease assays use DNA hairpins with a fluorophore group on the 5' and a quencher on the 3' end so that fluorescence increases as the oligonucleotide is degraded due to a separation of the fluorophore and the quencher48. While these assays allow one to characterize the kinetics of DNA-degrading proteins, they require previous knowledge of the enzyme's function and substrate and are also limited to enzymes that change the DNA conformation to cause a difference in dye binding. For this reason, endpoint assays that resolve individual nuclease products are still desirable to provide insight into DNA modifications caused by protein activity.
Here, a detailed procedure is presented for the design of fluorescently labeled DNA/RNA oligonucleotides that can be mixed and matched to generate substrates for testing the activity of novel nuclease, polymerase, and ligase enzymes. The validation of this basic set of oligonucleotide sequences simplifies experimental design and facilitates economical profiling of a wide range of enzymatic functionalities without needing to purchase a large number of bespoke substrates. A detailed procedure is provided for running a standard DNA-processing enzyme assay with these substrates, using the example of DNA ligase activity and modifications for assaying and analyzing nuclease and polymerase enzymes are described. In addition, a modified assay for determining the cofactor specificity of the DNA ligase enzyme with high accuracy is given, and dual-labeled probes are used to evaluate the assembly of multi-component ligations. Finally, modifications to the basic assay format are discussed to allow it to be used to determine protein-DNA interactions with the same substrates by the electrophoretic mobility shift assay (EMSA).
Access restricted. Please log in or start a trial to view this content.
Protocol
1. Design and purchase of oligonucleotides
NOTE: Design single-stranded oligonucleotides to be assembled and annealed into the desired duplexes. One or more of the strands in a duplex must bear a fluorescent moiety for tracking oligonucleotide processing by the enzyme of interest. A basis set of single-stranded sequences that can be assembled for a range of activities is provided in Table 1.
- Incorporate the specific modifications needed for the enzyme of interest as described below.
- For DNA ligase substrates (Figure 1): Assemble the simplest substrate from three oligonucleotides: a 5' phosphorylated donor strand (NL2), a 5' FAM-labeled acceptor strand (NL1), and a complement that bridges the two (NL3).
- Ensure strands providing the 5' terminus of the ligatable nick are phosphorylated before assembly of the substrate master mix in step 2. Order this as a modification on NL2 (as given in Table 1) or use enzymatic phosphorylation with T4 polynucleotide kinase after resuspending the oligonucleotides.
- Include 5'-terminal phosphorylation of NL6 and NL8, which comprise the complement of double-stranded breaks depicted in Figure 1A (NL6/NL7 and NL8/NL9) as this most closely resembles the natural substrate produced from a restriction endonuclease. Use a dual-labeled substrate to determine the relative extents of ligation for multi-part assemblies (see step 6).
- Alter the complement strand to produce mismatches (NL10) and gaps (NL11).
NOTE: Variations on the simple nicked substrate are depicted in Figure 1A. It is possible to use other sequences to produce an even wider range of mismatches or longer gaps by varying the underlined position. - Substitute DNA oligonucleotides for RNA oligonucleotides.
NOTE: Variations on the simple nicked substrate are depicted in Figure 1B. A wider range of DNA/RNA duplexes can be generated by additional combinations of the basis set given here to generate, for example, double-stranded breaks containing both RNA and DNA. An example of this variation is given in step 6 below, where a dual-label strategy is used.
- For DNA polymerase substrates: Assemble the oligonucleotides NL1 and NL3 listed in Table 1 to give a simple primer-extension assay. Investigate additional aspects of polymerase activity by introducing modifications into either the NL1 (primer) or NL3 (template) strands.
- Incorporate damaged base analogs into the NL3 oligonucleotide prior to position 20 to determine the ability to bypass damaged lesions on the template strand.
- Incorporate damaged base analogs into the NL1 oligonucleotide at position 20 to determine the ability to extend a damaged primer.
- Use either RNL1 or RNL3 in the duplex to investigate the extension of an RNA primer or the use of an RNA template.
- For nuclease substrates (Figure 2): Assemble oligonucleotides to give a non-exhaustive range of double and single-stranded substrates (Figure 2Ai) as well as a range of flapped and splayed junctions (Figure 2Aii) and damaged substrates (Figure 2B).
- To probe ribonuclease activities, iteratively substitute NL1, NL2, and NL3 with RNL1, RNL2, and RNL3. Use additional RNA versions of HJ5 and HJ6 to expand this set further.
- Use oligonucleotides MD5, MD6, and MD9 that have a centrally placed modification that mimics oxidative damage, an abasic repair intermediate, or a deamination product (Figure 2B). The substrates will detect cleavage of the strand at this position. Label the complement NL3 strand with an orthogonal fluorophore such as TAMRA to detect double-strand cleavage (see step 6).
- Use orthogonal labeling of the complement to detect double-stranded cutting at the mismatched sites on both the probe (NL5 and ND9) and the complement (MD10 and NL10) strands.
- For DNA ligase substrates (Figure 1): Assemble the simplest substrate from three oligonucleotides: a 5' phosphorylated donor strand (NL2), a 5' FAM-labeled acceptor strand (NL1), and a complement that bridges the two (NL3).
- Order synthetic oligonucleotides incorporating relevant fluorophores and other modifications from a commercial vendor.
NOTE: A 100 nM synthesis scale and HPLC purification subsequent to synthesis are suitable for the assays described.
2. Assembling and annealing nucleic acid duplexes
- Resuspension and dilution of oligonucleotides
- Before opening, centrifuge the lyophilized oligonucleotides in their 2 mL tubes at full speed in a benchtop centrifuge for 2-5 min to ensure the nucleic acid is on the bottom of the tube.
- Prepare a master stock of 100 µM by resuspending the oligonucleotides in TE buffer (10 mM tris(hydroxymethyl)aminomethane (Tris), 1 mM ethylenediaminetetraacetic acid (EDTA)). Ensure the oligonucleotides are thoroughly resuspended by repeated gentle vortexing and brief centrifugation at full speed.
- Prepare a 10 µM stock by diluting an aliquot of master stock with TE buffer. Dilute the 10 µM stock with ultrapure water (MQ water) to prepare working stocks with concentrations of 0.5 µM, 0.7 µM, or 2.5 µM as per Table 2.
- Assembling and annealing the reaction master mixes
- Use working stocks to make up the reaction master mixes using the combinations provided in Table 2 and volumes given in Table 3. For the standard DNA ligase assay and most other assays described here, the final buffer composition is 50 mM Tris pH 8.0, 50 mM NaCl, 10 mM Dithiothreitol (DTT) with 10 mM Mg as the divalent cation.
- Anneal the oligonucleotides in a PCR or microcentrifuge tube by heating at 95 °C for 5 min using a heating block or thermocycler. Allow to cool to room temperature for 30 min (volumes <1 mL) to 1 h (volumes >1 mL). For longer oligonucleotides (>40 nt), carry out slower cooling by using a thermocycler with a down ramp of 95 °C to 25 °C over 45 min, or float the tube containing annealing mixture in a 1 L beaker of boiling water and allow to cool to room temperature overnight.
- Add nucleotide cofactors and other heat-sensitive buffer components to the master mix after cooling to room temperature. Use the final reaction mixture directly for the assay by the addition of enzyme (see step 3 below) or store at -20 °C for future use.
3. Standard assay setup
- Assembly and initiation of the assay reaction
- Combine 22.5 µL of the substrate master mix of interest with 2.5 µL of the DNA ligase or other enzyme of interest in a PCR tube. Run reactions in duplicate or triplicate, especially if the results will be quantitated.
- Include a no-protein control (buffer only) in the assay samples. Include no cofactor controls at this point, if needed.
NOTE: Enzymes can be stored at -20 °C in 50% v/v glycerol, allowing them to be pipetted directly from solution. Ensure enzyme solutions with glycerol are well mixed prior to addition, either by pipetting to mix or by gentle vortexing.
- Immediately transfer the reactions to a PCR machine at 25 °C and incubate for 30 min. Vary the temperature and duration depending on the optimal conditions for enzyme activity.
- Quench the reactions by adding 5 µL of loading dye (95% formamide, 0.5 M Ethylenediaminetetraacetic acid (EDTA), bromophenol blue) and incubate at 95 °C for 5 min.
4. Analysis of assay results
- Prepare Tris-Borate-EDTA (TBE)-Urea PAGE gels as described below.
- Prepare a stock of 20% acrylamide, 7 M Urea, and 1x TBE solution. For the oligonucleotide set described here, use Acrylamide/Bis Solution in a 29:1 ratio for optimal resolution.
- For one gel, combine 10 mL of 20% acrylamide and 7 M urea solution with 100 µL of APS (10 %) and 3 µL of Tetramethylethylenediamine (TMED) and cast in a gel caster.
- After the gel solidifies, run the samples on the TBE urea gel at 45 - 55 °C.
- Pre-run the gel in 1x TBE buffer for 30 min at 10 mA per gel with external heating.
- Remove excess urea in the wells of the gel by flushing with 1x TBE using a pasture pipette.
- Load 10 µL of each reaction and run at 10 mA for 1.0-1.5 h with external heating.
- Visualize the gel on the imager with the correct settings for the chosen fluorophore. For FAM, use a filter set that gives excitation/emission at 495/519 nm, which is stored as a pre-set in most imagers.
- Quantify the band intensity of the product and substrate using image processing software with the imager, or an external program such as ImageJ49,50 and calculate the percentage of the product using the formula
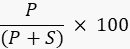
Where P is the integrated value of the product band, and S is the integrated area of the substrate band. In the case of the DNA ligase reaction example, the product band runs at 40 nucleotides (nt) and the substrate band at 20 nt.
5. De-adenylation of the DNA ligase to test cofactor specificity
- Preparation of reaction master mixes
- Prepare one set of the master mix containing the FAM-labeled NL1 oligonucleotide as described in Table 4. Prepare a second set containing the NL1 oligonucleotide with no FAM label, as described in Table 4.
- Separately, heat both DNA duplexes to 95 °C for 5 min and cool for 30 min to 1 h at 25 °C. Do not add nucleotide cofactor to either master mix.
- Assembly and initiation of de-adenylation reaction
- Prepare a single de-adenylation reaction for each cofactor type/ concentration to be tested by combining 10 µL of the unlabeled master mix with 2.5 µL of ligase enzyme.
- Prepare additional tubes as no-cofactor control and no protein control (2.5 µL of buffer added in place of enzyme).
- Incubate the reactions at a temperature specific to the enzyme's optimum activity, for 1-2 h. Incubation time can be increased if the enzyme is still adenylated.
- Run the ligation reaction with the cofactor.
- Add 10 µL of the labeled master mix and 2.5 µL of the desired nucleotide cofactors (e.g., ATP, NAD, ADP, or GTP) directly to the de-adenylated reaction (0.1-1 mM final concentration).
- Add 2.5 µL of reaction buffer to the no nucleotide cofactor control.
- Incubate the reactions for the same time period and temperature as previously used. Quench and visualize as described in step 4.
6. Using dual-labeled substrates for splinted ligation or multi-part assembly
- Design and purchase an oligonucleotide with a florescent moiety that has a different excitation/ emission spectrum to the fluorophore already used.
- In the set-up described here, use NL2 (TAMRA) oligonucleotide having 5-Carboxytetramethylrhodamine (TAMRA) on the 3' end (Table 1).
- Assemble the master mix as described below.
- Combine the components of the reaction described in step 2, including equimolar ratios of all oligonucleotides used in the assembly, as well as buffer and divalent cations.
- Anneal by heating at 95 °C for 5 min and cooling at 25 °C for 30 min - 1 h. Add the cofactor and the enzyme and incubate as described in step 3.
- Run and image the samples as described in step 4 using the appropriate channels for the fluorophore pair in the substrate. In the case of FAM and TAMRA, these are the Fluorescein (FITC) and Tetramethyl rhodamine (TRITC) channels present on most imagers.
7. Evaluation of DNA binding by Electrophoretic Mobility Shift Assay (EMSA) on native gel
- Prepare a 10% native TBE PAGE gel as described below.
- Combine 2.5 mL of 40% acrylamide, 1 mL of 10x TBE, 100 µL of 10% APS, 3 µL of TMED, and 6.5 mL of MQ water and cast in a gel caster.
- Assemble the binding reaction as described below.
- Assemble the EMSA substrate according to Table 5 so that EDTA (10 mM) is included and metal ions are omitted.
- Combine 20 µL of the EMSA substrate master mix with 5 µL of the protein in a PCR tube. Include a no protein control sample. Incubate for 30 min at 25 °C.
- Analyze by native electrophoresis as described below.
- Add 5 µL of 5x native loading dye (100 mM EDTA, 0.25 % bromophenol blue, 25% v/v glycerol, and MQ water up to 1 mL) to the samples.
- Load on the prepared gel and run at 60 V for 2-3 h with cooling by water circulation until the dye front is a few cm above the end of the gel.
- Visualize and analyze gels as described in step 4.
Access restricted. Please log in or start a trial to view this content.
Results
Ligation by DNA ligase
DNA ligase enzymatic activity will result in an increase in the size of the fluorescently labeled oligonucleotide when visualized on a urea PAGE gel. In the case of the substrates for both DNA- and RNA-ligation listed in Table 2, this corresponds to a doubling in size from 20 nt to 40 nt (Figure 3A). Optimal enzyme activity can be determined by changing conditions such as temperature, protein concentration, or incubation time (
Access restricted. Please log in or start a trial to view this content.
Discussion
Critical steps in the protocol
Oligonucleotide design and purchase: When purchasing the oligonucleotides for duplex formation, it is essential to consider sequence design. It is recommended to use an oligo analyzer tool to predict properties of the nucleotide sequence, such as GC content, melting temperature, secondary structure, and dimerization potential, before ordering57.
Assembly and annealing of nucleic acid duplexes: When preparing RNA/RNA-...
Access restricted. Please log in or start a trial to view this content.
Disclosures
SEG and UR are employees of ArcticZymes Technologies AS which distributes the R2D ligase. AW, ER-S and RS have no competing interests.
Acknowledgements
AW is supported by a Rutherford Discovery Fellowship (20-UOW-004). RS is the recipient of a New Zealand Post Antarctic Scholarship. SG and UR acknowledge the Chemical Institute at the University of Tromsø - The Norwegian Arctic University for technical support.
Access restricted. Please log in or start a trial to view this content.
Materials
| Name | Company | Catalog Number | Comments |
| 30% Acrylamide/Bis Solution (29:1) | BioRad | 1610156 | |
| Adenosine triphosphate (ATP) | Many suppliers | ||
| Ammonium persulfate (APS) | Many suppliers | ||
| Benchtop centrifuge | Many suppliers | ||
| Borate | Many suppliers | ||
| Bromophenol blue | Many suppliers | ||
| Dithiothreitol (DTT) | Many suppliers | ||
| Electrophoresis system with circulating water bath | Many suppliers | ||
| Ethylenediaminetetraacetic acid (EDTA) | Many suppliers | ||
| Fluoresnence imager, e.g. iBright FL1000 | Thermo Fisher Scientific | A32752 | |
| Formamide | Many suppliers | ||
| Gel casting system | Many suppliers | ||
| Heating block | Many suppliers | ||
| Magnesium Chloride | Many suppliers | Other metal ions may be preferred depending on the protein studied | |
| Microcentrifuge tubes (1.5 mL) | Many suppliers | ||
| Micropipettes and tips | Many suppliers | 1 mL, 0.2 mL, 0.02 mL, 0.002 mL | |
| Nicotinamide adenine dinucleotide (NAD+) | Many suppliers | ||
| Oligonucleotides | Integrated DNA Technologies | NA | Thermo Fisher, Sigma-Aldrich, Genscript and others also supply these |
| pasture pipette | Many suppliers | ||
| PCR thermocycler | Many suppliers | ||
| PCR tubes | Many suppliers | ||
| RNAse away | ThermoFisher | 7002PK | Only needed when working with RNA oligos |
| RNase AWAY | Merck | 83931-250ML | Surfactant for removal of RNAse contamination on surfaces |
| RNAse-free water | New England Biolabs | B1500L | Only needed when working with RNA oligos |
| Sodium Chloride | Many suppliers | ||
| SUPERase IN RNase inhibitor | Thermo Fisher Scientific | AM2694 | Broad spectrum RNAse inhibitir (protein-based) |
| SYBR Gold | Thermo Fisher Scientific | S11494 | This may be used to post-stain gels and visualise unlabelled oligonucleotides |
| Tetramethylethylenediamine (TMED) | Many suppliers | ||
| Tris, or tris(hydroxymethyl)aminomethane | Many suppliers | ||
| Ultrapure water (Milli-Q) | Merck | ||
| urea | Many suppliers | ||
| Vortex | Many suppliers |
References
- Gao, Y., et al. Structures and operating principles of the replisome. Science. 363 (6429), 7003(2019).
- Yang, W., Gao, Y. Translesion and repair DNA polymerases: Diverse structure and mechanism. Annu Rev Biochem. 87, 239-261 (2018).
- Lohman, T. M., Fazio, N. T. How does a helicase unwind DNA? Insights from RecBCD Helicase. Bioessays. 40 (6), e1800009(2018).
- Ahdash, Z., et al. Mechanistic insight into the assembly of the HerA-NurA helicase-nuclease DNA end resection complex. Nucleic Acids Res. 45 (20), 12025-12038 (2017).
- Wozniak, K. J., Simmons, L. A. Bacterial DNA excision repair pathways. Nat Rev Microbiol. 20 (8), 465-477 (2022).
- Zhang, L., Jiang, D., Wu, M., Yang, Z., Oger, P. M. New insights into DNA repair revealed by NucS endonucleases from hyperthermophilic Archaea. Front Microbiol. 11, 1263(2020).
- Saathoff, J. H., Kashammer, L., Lammens, K., Byrne, R. T., Hopfner, K. P. The bacterial Mre11-Rad50 homolog SbcCD cleaves opposing strands of DNA by two chemically distinct nuclease reactions. Nucleic Acids Res. 46 (21), 11303-11314 (2018).
- Williamson, A., Leiros, H. S. Structural insight into DNA joining: from conserved mechanisms to diverse scaffolds. Nucleic Acids Res. 48 (15), 8225-8242 (2020).
- Caglayan, M. Interplay between DNA polymerases and DNA ligases: Influence on substrate channeling and the fidelity of DNA ligation. J Mol Biol. 431 (11), 2068-2081 (2019).
- Shuman, S. DNA ligases: Progress and prospects. J Biol Chem. 284 (26), 17365-17369 (2009).
- Lohman, G. J., Tabor, S., Nichols, N. M. DNA ligases. Curr Prot Mol Biol. , Chapter 3, Unit 3.14 (2011).
- Rittié, L., Perbal, B. Enzymes used in molecular biology: a useful guide. J Cell Commun Signal. 2 (1-2), 25-45 (2008).
- Chandrasegaran, S., Carroll, D. Origins of programmable nucleases for genome engineering. J Mol Biol. 428 (5), Part b 963-989 (2016).
- Aschenbrenner, J., Marx, A. DNA polymerases and biotechnological applications. Curr Opin Biotechnol. 48, 187-195 (2017).
- Loenen, W. A. M., Dryden, D. T. F., Raleigh, E. A., Wilson, G. G., Murray, N. E. Highlights of the DNA cutters: a short history of the restriction enzymes. Nucleic Acids Res. 42 (1), 3-19 (2013).
- Voytas, D., Ke, N. Detection and quantitation of radiolabeled proteins and DNA in gels and blots. Curr Protoc Immunol. , Appendix 3 (A) (2002).
- Phillips, D. H. Detection of DNA modifications by the 32P-postlabelling assay. Mutat Res. 378 (1-2), 1-12 (1997).
- Huang, C., Yu, Y. T. Synthesis and labeling of RNA in vitro. Curr Prot Mol Biol. , Chapter 4, Unit 4.15 (2013).
- Ballal, R., Cheema, A., Ahmad, W., Rosen, E. M., Saha, T. Fluorescent oligonucleotides can serve as suitable alternatives to radiolabeled oligonucleotides. J Biomol Tech. 20 (4), 190-194 (2009).
- Anderson, B. J., Larkin, C., Guja, K., Schildbach, J. F. Using fluorophore-labeled oligonucleotides to measure affinities of protein-DNA interactions. Meth Enzymol. 450, 253-272 (2008).
- Liu, W., et al. Establishment of an accurate and fast detection method using molecular beacons in loop-mediated isothermal amplification assay. Sci Rep. 7 (1), 40125(2017).
- Ma, C., et al. Simultaneous detection of kinase and phosphatase activities of polynucleotide kinase using molecular beacon probes. Anal Biochem. 443 (2), 166-168 (2013).
- Li, J., Cao, Z. C., Tang, Z., Wang, K., Tan, W. Molecular beacons for protein-DNA interaction studies. Meth Mol Biol. 429, 209-224 (2008).
- Yang, C. J., Li, J. J., Tan, W. Using molecular beacons for sensitive fluorescence assays of the enzymatic cleavage of nucleic acids. Meth Mol Biol. 335, 71-81 (2006).
- Nikiforov, T. T., Roman, S. Fluorogenic DNA ligase and base excision repair enzyme assays using substrates labeled with single fluorophores. Anal Biochem. 477, 69-77 (2015).
- Pergolizzi, G., Wagner, G. K., Bowater, R. P. Biochemical and structural characterisation of DNA ligases from bacteria and Archaea. Biosci Rep. 36 (5), 00391(2016).
- Martin, I. V., MacNeill, S. A. ATP-dependent DNA ligases. Genome Biol. 3 (4), REVIEWS3005 (2002).
- Bilotti, K., et al. Mismatch discrimination and sequence bias during end-joining by DNA ligases. Nucleic Acids Res. 50 (8), 4647-4658 (2022).
- Bauer, R. J., et al. Comparative analysis of the end-joining activity of several DNA ligases. PLoS One. 12 (12), e0190062(2017).
- Lohman, G. J. S., Zhang, Y., Zhelkovsky, A. M., Cantor, E. J., Evans, T. C. Efficient DNA ligation in DNA-RNA hybrid helices by Chlorella virus DNA ligase. Nucleic Acids Res. 42 (3), 1831-1844 (2014).
- Bullard, D. R., Bowater, R. P. Direct comparison of nick-joining activity of the nucleic acid ligases from bacteriophage T4. Biochem J. 398 (1), 135-144 (2006).
- Magnet, S., Blanchard, J. S. Mechanistic and kinetic study of the ATP-dependent DNA ligase of Neisseria meningitidis. Biochemistry. 43 (3), 710-717 (2004).
- Williamson, A., Grgic, M., Leiros, H. S. DNA binding with a minimal scaffold: structure-function analysis of Lig E DNA ligases. Nucleic Acids Res. 46 (16), 8616-8629 (2018).
- Lohman, G. J., et al. A high-throughput assay for the comprehensive profiling of DNA ligase fidelity. Nucleic Acids Res. 44 (2), e14(2016).
- Kim, J., Mrksich, M. Profiling the selectivity of DNA ligases in an array format with mass spectrometry. Nucleic Acids Res. 38 (1), e2(2010).
- Tang, Z. W., et al. Real-time monitoring of nucleic acid ligation in homogenous solutions using molecular beacons. Nucleic Acids Res. 31 (23), e148(2003).
- Yang, W. Nucleases: diversity of structure, function and mechanism. Q Rev Biophys. 44 (1), 1-93 (2011).
- Marti, T. M., Fleck, O. DNA repair nucleases. Cell Mol Life Sci. 61 (3), 336-354 (2004).
- Wang, B. B., et al. Review of DNA repair enzymes in bacteria: With a major focus on AddAB and RecBCD. DNA Repair. 118, 103389(2022).
- Pidugu, L. S., et al. Structural insights into the mechanism of base excision by MBD4. J Mol Biol. 433 (15), 167097(2021).
- Roberts, R. J. How restriction enzymes became the workhorses of molecular biology. Proc Natl Acad Sci U S A. 102 (17), 5905-5908 (2005).
- Miller, J. C., et al. An improved zinc-finger nuclease architecture for highly specific genome editing. Nat Biotechnol. 25 (7), 778-785 (2007).
- Kim, H., Kim, J. S. A guide to genome engineering with programmable nucleases. Nat Rev Genet. 15 (5), 321-334 (2014).
- Takemoto, N., Numata, I., Su'etsugu, M., Miyoshi-Akiyama, T. Bacterial EndoMS/NucS acts as a clamp-mediated mismatch endonuclease to prevent asymmetric accumulation of replication errors. Nucleic Acids Res. 46 (12), 6152-6165 (2018).
- Reardon, J. T., Sancar, A. Molecular anatomy of the human excision nuclease assembled at sites of DNA damage. Mol Cell Biol. 22 (16), 5938-5945 (2002).
- Kunkel, T. A., Soni, A. Exonucleolytic proofreading enhances the fidelity of DNA synthesis by chick embryo DNA polymerase-gamma. J Biol Chem. 263 (9), 4450-4459 (1988).
- Sheppard, E. C., Rogers, S., Harmer, N. J., Chahwan, R. A universal fluorescence-based toolkit for real-time quantification of DNA and RNA nuclease activity. Sci Rep. 9 (1), 8853(2019).
- Li, J. J., Geyer, R., Tan, W. Using molecular beacons as a sensitive fluorescence assay for enzymatic cleavage of single-stranded DNA. Nucleic Acids Res. 28 (11), e52(2000).
- Schneider, C. A., Rasband, W. S., Eliceiri, K. W. NIH Image to ImageJ: 25 years of image analysis. Nat Meth. 9 (7), 671-675 (2012).
- Sharma, J. K., et al. Methods for competitive enrichment and evaluation of superior DNA ligases. Meth Enzymol. 644, 209-225 (2020).
- Rzoska-Smith, E., Stelzer, R., Monterio, M., Cary, S. C., Williamson, A. DNA repair enzymes of the Antarctic Dry Valley metagenome. Front Microbiol. 14, 1156817(2023).
- Williamson, A., Pedersen, H. Recombinant expression and purification of an ATP-dependent DNA ligase from Aliivibrio salmonicida. Protein Expres Purif. 97 (0), 29-36 (2014).
- Akey, D., et al. Crystal structure and nonhomologous end-joining function of the ligase component of Mycobacterium DNA ligase D. J Biol Chem. 281 (19), 13412-13423 (2006).
- Kim, D. J., et al. ATP-dependent DNA ligase from Archaeoglobus fulgidus displays a tightly closed conformation. Acta Crystallogr Sect F Struct Biol Cryst Commun. 65, Pt 6 544-550 (2009).
- Nishida, H., Kiyonari, S., Ishino, Y., Morikawa, K. The closed structure of an archaeal DNA ligase from Pyrococcus furiosus. J Mol Biol. 360 (5), 956-967 (2006).
- Gundesø, S., et al. R2D ligase: Unveiling a novel DNA ligase with surprising DNA-to-RNA ligation activity. Biotechnol J. 19 (3), e2300711(2024).
- Hendling, M., Barišić, I. In silico design of DNA oligonucleotides: Challenges and approaches. Comput Struct Biotechnol J. 17, 1056-1065 (2019).
- Green, M. R., Sambrook, J. How to win the battle with RNase. Cold Spring Harb Prot. 2019 (2), 10.1101/pdb.top101857 (2019).
- Summer, H., Grämer, R., Dröge, P. Denaturing urea polyacrylamide gel electrophoresis (Urea PAGE). J Vis Exp. (32), e1485(2009).
- Smith, D. R. Gel Electrophoresis of DNA. Mol Biometh Handbook. , Humana Press. Totowa, NJ. 17-33 (1998).
- Lorenz, T. C. Polymerase chain reaction: basic protocol plus troubleshooting and optimization strategies. J Vis Exp. (63), e3998(2012).
- Rousseau, M., et al. Characterisation and engineering of a thermophilic RNA ligase from Palaeococcus pacificus. Nucleic Acids Res. 52 (7), 3924-3937 (2024).
- Kestemont, D., Herdewijn, P., Renders, M. Enzymatic synthesis of backbone-modified oligonucleotides using T4 DNA ligase. Curr Prot Chem Biol. 11 (2), e62(2019).
- Farell, E. M., Alexandre, G. Bovine serum albumin further enhances the effects of organic solvents on increased yield of polymerase chain reaction of GC-rich templates. BMC Res Notes. 5, 257(2012).
- Nazarenko, I., Pires, R., Lowe, B., Obaidy, M., Rashtchian, A. Effect of primary and secondary structure of oligodeoxyribonucleotides on the fluorescent properties of conjugated dyes. Nucleic Acids Res. 30 (9), 2089-2195 (2002).
Access restricted. Please log in or start a trial to view this content.
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved