Research Article
Herbal Munziq Ameliorates Myocardial Ischemia-Reperfusion Injury by Inhibiting Inflammation
In This Article
Summary
This study investigates the cardioprotective effects of Munziq, a traditional Uyghur herbal preparation, on myocardial ischemia-reperfusion injury (MIRI) in rats with abnormal body fluid. Through comprehensive experimental methods, we demonstrate Munziq's potential to mitigate MIRI by suppressing the NF-κB signaling pathway.
Abstract
The objective of this study was to investigate the cardioprotective effects of Munziq on abnormal body fluid myocardial ischemia-reperfusion injury (MIRI) and its underlying mechanism.Normal rats and rats with abnormal body fluid (ABF) were pre-treated with Munziq for 21 days. Following this, MIRI models were established. Histopathological changes and myocardial ultrastructure changes were observed by Hematoxylin and Eosin (HE)staining and transmission electron microscopy to observe pathological manifestations of myocardial injury. Serum CK-MB, cTn-T, and ICAM-1 levels were detected by Enzyme-Linked Immunosorbent Assay (ELISA) to observe myocardial injury-related markers. The levels of IL-1β, IL-6, and TNF-α in serum and myocardial tissue were also detected by ELISA to observe the anti-inflammatory effect. The expression levels of NF-κB signaling pathway-related proteins NIK, IKKα, Pikα, and p65 were detected by Western blot analysis. The results showed that myocardial injury in the ABF MIRI group was more severe compared to the control MIRI group. Munziq pretreatment has the potential to mitigate the pathological changes induced by ischemia-reperfusion injury and could protect cardiac function. Protein levels of the NF-κB pathway and downstream effectors IL-1β, IL-6, and TNF-α were significantly up-regulated in the MIRI group while down-regulated in the Munziq group. Interestingly, there was more activation of the NF-κB signaling pathway and higher levels of downstream inflammatory cytokines in the ABF MIRI group. The results suggest that MIRI was more severe in ABF. Munziq has cardioprotective effects in ischemia and reperfusion injury. This protective effect may be acted by suppressing the NF-κB signaling pathway.
Introduction
Myocardial ischemia is a condition where the myocardium does not receive adequate blood flow, primarily caused by stenosis or thrombosis of the coronary arteries1, which can lead to fatal outcomes for patients2,3. Since myocardial metabolism is almost exclusively aerobic and contains very limited glycogen stores, it is essential to restore blood supply promptly, primarily through PCI or intravenous thrombolysis. While effective myocardial reperfusion is crucial for improving the prognosis of ischemic myocardium, it also introduces the risk of myocardial ischemia-reperfusion injury (MIRI)4,5,6. MIRI is a significant challenge that impacts the efficacy of myocardial reperfusion therapies7. Multiple factors and mechanisms contribute to the development of MIRI. For instance, in endothelial cells, reperfusion induces an accumulation of reactive oxygen species (ROS) and a depletion of free radical scavengers, indicating the presence of oxidative stress4,8. This oxidative stress may subsequently trigger an inflammatory response, leading to enhanced release of inflammatory factors, increased adhesion molecule production, and recruitment of leukocytes9,10,11. The nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) pathway plays a crucial role in the inflammatory response during myocardial ischemia/reperfusion injury12. The mammalian NF-κB transcription factor family includes five members: NF-κB1 (also known as p105/p50), NF-κB2 (also known as p100/p52), p65 (also referred to as RELA), RELB (the homolog of the V-Rel reticuloendotheliosis viral oncogene), and c-REL13,14. Inhibition of the NF-κB pathway has been shown to alleviate ischemia/reperfusion injury in various tissues, including the myocardium12,15, intestine16, ovary17, brain18,19, kidneys20, and liver21. Notably, the NF-κB signaling pathway has been extensively documented as a pivotal mediator in the myocardial response to ischemia and reperfusion12,14, possible mechanisms include promoting inflammatory responses, regulating the expression of cell adhesion molecules, participating in oxidative stress reactions, and influencing cell death and survival pathways12,20,22,23,24,25. Therapeutic interventions aimed at attenuating NF-κB activation have shown significant potential in alleviating MIRI23,26 .
Uygur medicine, an integral part of traditional Chinese medicine, is founded on the theory of four humors: fire, air, water, and earth. These humors give rise to bodily fluids such as blood, phlegm, yellow bile, and black bile27,28. The maintenance of dynamic homeostasis among these four bodily fluids is crucial for the overall health of the human body. Any imbalance in these fluids, referred to as unbalanced body fluids, can result in the onset of diseases. Among the various unbalanced body fluids, the predominant one is known as abnormal body fluid (ABF), also recognized as abnormal Savda syndrome (ASS)27,28. Munziq, a traditional Uyghur medicine extensively employed by Uyghur physicians, is prescribed for the treatment of abnormal body fluid. It is an herbal medicinal preparation comprising ten different medicinal species, as provided in the package insert, including Cordia dichotoma Forst. f., Anchusaitalica Retz., Glycyrrhiza uralensis Fisch., Adiantum capillusveneris L., Euphorbia humifusa Willd., Ziziphus jujuba Mill., Lavandula angustifolia Mill., Foeniculum vulgare Mill., Melissa officinalis L., and Alhagi pseudoalhagi Desv29. Munziq was approved by the State Food and Drug Administration in 2003 under the code number Z65020166. Its active chemical components encompass brass, phenols, organic acids, amino acids, saponin, sugar, and others. Munziq exhibits multiple effects, including antioxidant, anti-inflammatory, immune-regulatory, anti-platelet aggregation, and antithrombotic effects27,28,30.
Our previous studies have shown that Munziq medicine can alleviate myocardial ischemia/reperfusion injury (MIRI), though the specific mechanisms remain unclear. Myocardial protective effects of Munziq in myocardial ischemia-reperfusion injury rats with abnormal Savda syndrome were shown. Inhibition of nuclear factor kappa b pathway protects myocardial ischemia/reperfusion injury in rats under treatment with Fufang Munziq granule (Munziq) 27,28,30. However, there is limited research investigating the role and mechanism of Munziq in MIRI.
This study aims to investigate the cardioprotective effects of Munziq on myocardial ischemia-reperfusion injury (MIRI) in rats with abnormal body fluid (ABF) and to explore the underlying mechanisms, particularly focusing on the NF-κB signaling pathway. The hypothesis proposed in this study is that Munziq pretreatment can mitigate the pathological changes induced by MIRI and protect cardiac function, potentially by suppressing the NF-κB signaling pathway and downstream inflammatory responses. The hypothesis is tested by establishing sham, ischemia-reperfusion injury (MIRI), and ischemia-reperfusion injury + Munziq models in both control and ABF groups. Cardiac function, inflammation-related indicators, and proteins associated with the NF-κB pathway are monitored.
Protocol
Adult male Sprague-Dawley (SD) rat weighing 200-220 g was used in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals. Animal Experimental Center of Xinjiang Medical University. Animal models with abnormal body fluid (ABF) were provided by the Experimental Animal Center of Xinjiang Medical University. Rats were kept in a dry-cold environment as previously described, which is essential for establishing the abnormal body fluid (ABF) model in rats, according to traditional Uyghur medicine theory and as demonstrated in previous studies29,33. All animal experiments were conducted according to the ethical guidelines of Xinjiang Medical University and carried out in accordance with ARRIVE guidelines. The protocol for vertebrate animal (rat) studies was approved by the institutional ethical committee of Xinjiang Medical University (IACUC-20200318-16).
1. Animal grouping, drug administration, and MIRI model establishment
- Randomly assign rats to the following six groups: i) control sham group, ii) control MIRI group, iii) ABF sham group, iv) ABF MIRI group, v) control MIRI + Munziq group and vi) ABF MIRI + Munziq group.
- House the rats in the ABF group in a controlled environment within climate boxes set to a temperature of 6 °C ± 1 °C and a relative humidity range of 25% to 32.8%. Provide the rats with ordinary feed mixed with dry cold food, namely barley and coriander seeds at a ratio of 7:1.5:1.5, and apply this method for 21 days to establish the ABF model29,33.
- Administer by intragastric administration 5 g/kg Munziq (5.0 g of Munziq dissolved in 1 mL distilled water) to the Munziq group for 21 days prior to the MIRI surgery, which is double the effective clinical dose. For non-ABF animals, such as the Control Sham, Control MIRI, and Control MIRI + Munziq groups, administer an equal volume of normal saline to the sham group and MIRI group and provide intragastric administration of Munziq for the Munziq group.
- After 21 days of pretreatment, establish the MIRI model.
- Conduct the surgery in a dedicated animal operating room equipped with sterile materials to ensure a sterile environment. Anesthetize the rats with an intraperitoneal injection (IP) of sodium pentobarbital (35 mg/kg); observe muscle relaxation, responsiveness to external stimuli, and respiratory rate and depth to evaluate the anesthetic effect. Monitor these parameters every 15 min throughout the procedure. If signs of inadequate anesthesia appear (muscle tension, noticeable response to stimuli, increased or shallow respiratory rate), administer a supplemental dose of 20 mg/kg. Maintain anesthesia throughout the entire procedure until final blood collection. During the procedure, maintain the rat's body temperature at 37 °C using a heating pad with continuous monitoring via a rectal temperature probe. Apply vet ointment to the eyes to prevent dryness during anesthesia.
- Perform a tracheostomy for ventilator-assisted breathing. Before opening the chest to expose the heart, wash the surgical site with soapy water, shave the surgical area, and clean the area with antiseptic solutions such as chlorhexidine and iodine. Use sterile instruments for the procedure.
- To open the chest and expose the heart, use standard sterile thoracic surgical instruments, including scissors, forceps, and retractors. Open the chest wall and expose the heart. Identify the left anterior descending (LAD) artery under direct visualization. It is a major branch of the left coronary artery that lies on the surface of the heart. Use a 6-0 suture (4-5 cm), ligate the LAD (left anterior descending) for 30 min to induce regional ischemia. Determine effective LAD occlusion by observing a pale color in the myocardium.
- After 30 min of ligation, release the ligature and perform reperfusion for 120 min. Determine the reperfusion of myocardium when it recovers to a bright red color. In rats with sham operation, perform the same procedure but without ligation of LAD.
- After reperfusion, the rats remained under anesthesia induced by sodium pentobarbital (35 mg/kg, ip)34. Once the rats are in deep anesthesia, collect 1-2 mL of blood samples from the abdominal aorta using a vacuum blood collection tube.
- After blood sample collection from the abdominal aorta, euthanize the rats by cervical dislocation while still under deep anesthesia. Collect the myocardium tissue from the infarct area in the left ventricle using sterile forceps and scissors, ensuring to take only the tissue that appears pale and damaged, which indicates the infarcted region. Place the collected tissue in a sterile container for further analysis.
2. Sample collection
- Tissue sample collection: Using sterile scissors and a sterile blade, transect the heart horizontally into two halves along the mid-point of the left ventricular long axis, perpendicular to the direction of the heart. Divide one half of the apical part into two portions: Preserve one in 4% paraformaldehyde for morphological examination at room temperature or 4 °C for 2-24 h, and the other in glutaraldehyde for electron microscopy at 4 °C for 1-4 h.
- Divide the base part of the heart, including the ischemic and non-ischemic areas, into two portions: Place one portion into a cryovial, rapidly freeze the tissue in liquid nitrogen, and then transfer it to a -80 °C ultra-low temperature freezer for molecular biology testing. Use the other fresh portion for the detection of tissue reactive oxygen species (ROS) levels and mitochondrial extraction.
- Serum sample collection: At the endpoint of the experiment, draw 1-2 mL of venous blood from the inferior vena cava, centrifuge at 1000 x g for 10 min, and store the serum in a -80 °C ultra-low-temperature freezer after separation.
3. HE staining and transmission electron microscopy observation
- Perform HE staining (hematoxylin-eosin staining) on the ventricular myocardium according to routine procedure35. Fix the tissues in 4% paraformaldehyde for 24 h. Embed the samples in paraffin, cut them into 4 µm thick sections, and stain them using the hematoxylin-eosin staining method according to the protocol. Randomly select five fields to observe histopathological changes under the microscope.
- Process myocardial tissues from rats that have been fixed in formalin, from each group, for HE staining experiments following the steps below.
- Place the tissue sections in a 65 °C incubator for baking for 1.5 to 2.
- Immerse the tissue sections in xylene for 10 min, replace the xylene, and immerse again for another 10 min. Sequentially immerse in anhydrous alcohol I and II for 5 min each, followed by 95%, 90%, 80%, and 70% alcohol, and distilled water for 5 min each.
- Stain with hematoxylin for 3 min. Perform acidic differentiation with hydrochloric acid added in alcohol for a few seconds (1-2 s). Terminate differentiation in tap water for 5 min.
- Immerse in distilled water, 70%, 80%, 90%, and 95% alcohol for 3 min each, and anhydrous alcohol I and II for 5 min each.
- Stain with 0.5% eosin in ethanol for 1 min, rinse the sections in 95% ethanol to remove excess red color, then immerse in anhydrous ethanol for 5 min. Immerse in xylene I and II for 5 min each.
- Mount with neutral balsam. Observe pathological changes of the tissue under the microscope.
4. ELISA detection of blood cytokines and cardiac injury indexes
- Obtain rat serum sample from peripheral blood by centrifuging at 1000 x g for 10 min, and store at −80 °C. Detect levels of Cardiac injury indices such as CK-MB, cTn-T, ICAM-1 and inflammatory cytokines such as IL-1β, IL-6, TNF-α in serum using ELISA kits according to the manufacturers' instructions and as described below.
- Allow all reagents to equilibrate to room temperature (18-25 °C) for at least 30 min, prepare reagents according to the manufacturers' instructions, and have them ready for use.
- Set up standard and sample wells, where the standard wells contain known concentrations of standard solutions provided in the assay kit. These are used to establish a standard curve during the experiment to quantify the specific analyte concentration in the samples being tested. Add 100 µL of standard or sample to each well, gently mix by shaking, cover with a plate seal, and incubate at 37 °C for 2 h.
- Discard the liquid, dry by decanting, and do not wash. Add 100 µL of biotin-labeled antibody (Pre-diluted 1:100) working solution to each well, cover with a new plate seal, and incubate at 37 °C for 1 h.
- Discard the liquid from the wells, dry by decanting, and wash the plate 3x. Soak for 2 min per wash, 200 µL per well, and dry by decanting.
- Add 100 µL of horseradish peroxidase-labeled streptavidin working solution to each well, cover with a new plate seal, and incubate at 37 °C for 1 h.
- Discard the liquid from the wells, dry by decanting, and wash the plate 5x. Soak for 2 min per wash, 200 µL per well, and dry by decanting.
- Sequentially add 90 µL of substrate solution to each well, and develop the color in the dark at 37 °C for 15-30 min.
- Sequentially add 50 µL of stop solution to each well to terminate the reaction. Within 5 min after stopping the reaction, measure the optical density (OD values) of each well in sequence at 450 nm using a microplate reader.
5. Measurement of MDA, NO and LDH Level
- After reperfusion, collect myocardium tissue (5 mm x 7 mm) from infarct area in the left ventricle. Detect LDH (lactate dehydrogenase) and MDA (malondialdehyde) using the LDH Assay kit and MDA Assay kit, following the manufacturer's instructions. Detect NO at 550 nm using the Nitric Oxide (NO) assay kit according to the manufacturer's instructions.
6. Western blot analysis
- Extract total proteins from rat ventricular tissues using RIPA lysis. Determine protein concentration using the BCA Protein Assay Kit.
- To preserve intracellular proteins and prevent cellular degradation, immerse the samples in liquid nitrogen to rapidly freeze them. Subsequently, retrieve the frozen samples and ground them into a powder, take approximately 100 mg of the sample, and add them to a pre-cooled 1.5 mL centrifuge tube. Add 400 µL of RIPA lysis buffer (supplement with protease inhibitors and a broad-spectrum phosphatase inhibitor), then thoroughly mix and allow the mixture to stand at 4 °C for 60 min.
- Centrifuge the mixture at 1000 x g for 15 min at 4 °C. Collect the supernatant and measure the protein concentration using the BCA method.
- Dilute BSA standards with a diluent that matches the buffer system of the samples,according to Table 1.
- Prepare reagents according to the manufacturer's instructions. Based on the number of samples, mix reagents A and B in a volume ratio of 50:1 to prepare an adequate amount of BCA working solution and mix thoroughly. Before preparing the BCA working solution, shake reagent A to mix well.
- Take 20 µL of freshly prepared BSA standard solution and diluted samples (10-fold diluted) from Table 1 and add them to a 96-well plate.
- Add 200 µL of BCA working solution to each well and mix thoroughly. Seal the plate, incubate at 37 °C for 30 min, then cool to room temperature or place at room temperature for 2 min.
- Measure the absorbance at 562 nm using a microplate reader and calculate the protein concentration in the samples based on the standard curve.
- Add an appropriate amount of 5x SDS-PAGE loading buffer (containing β-mercaptoethanol) to the samples, heat treat at 100 °C in boiling water for 5 min to fully denature the proteins, centrifuge at 1,000 x g for 5 min, and take the supernatant for use.
- The formula in Table 2 outlines the preparation of 15%, 12%, and 8% separating gels, as well as a 5% stacking gel. Prepare the solutions accordingly. Add the separating gel solution to a height of 2/3 in the gel cassette, overlay it with distilled water, and let it stand at room temperature for 40 min. Then, add the stacking gel to fill the cassette, insert the comb, and let it sit for 10 min.
- Load 9 µL of pre-stained protein marker into each well, and load 50 µg of sample protein per well.
- Apply a constant voltage of 80 V until the bromophenol blue reaches the separating gel, then apply a constant voltage of 100 V for 90 min. Stop the electrophoresis when the bromophenol blue has migrated to the lower part of the gel.
- After the completion of SDS-PAGE, immerse the PVDF membrane in methanol for 10 s, rinse in distilled water for 1 min, and then soak the polyacrylamide gel, filter paper, and the treated PVDF membrane in transfer buffer for 10 min.
- Assemble the transfer sandwich with the black side of the clips facing down, followed by a sponge-filter paper-gel-PVDF membrane-filter paper-sponge-transparent side of the clips. Place the clips into the transfer cassette, ensuring that the black side of the clip faces the black side of the cassette and the white side faces the red side. Perform the transfer at a constant voltage of 100 V and use a PVDF membrane with a transfer time of 60 min.
- After the transfer, wash the PVDF membrane with water 3x for 5 min each. Block the membrane with a blocking solution containing 5% non-fat dry milk for 1 h, then wash with TBST 3x for 5 min each.
- Dilute the primary antibody with TBST according to the dilution ratios listed in Table 3 and incubate at 4 °C overnight. The primary antibodies include anti-NF-κB p65 antibody, anti-NF-κB Inducing Kinase (NIK) antibody, anti-IKKα antibody, and anti-β-actin antibody.
- Rinse the membrane 3x with 1x TBST for 5 min each. Add the appropriately diluted secondary antibody (as per Table 3) and incubate at room temperature for 1 h. The secondary antibodies are HRP-conjugated goat anti-mouse IgG and HRP-conjugated goat anti-rabbit IgG.
- Rinse the membrane again with 1x TBST 3x for 5 min each. Mix the developing solutions A and B, add 2 mL to the membrane, and detect and photograph using the mini chemiluminescence instrument.
- Develop the membrane using enhanced chemiluminescence plus reagent. Scan the developed film using the imaging system. Analyze the Western blot images using software. Use β-actin as the loading control. Calculate the relative protein level based on the grey value of β-actin.
7. Real-time PCR analysis (qRT-PCR)
- Total RNA extraction
- Prepare 0.1% DEPC Solution and 70% DEPC-treated Alcohol according to the manufacturer's instructions. Treat all sizes of microcentrifuge tubes and various types of pipette tips with 0.1% DEPC solution. Soak overnight, then autoclave.
- Collect cells in the logarithmic growth phase with good growth status. Discard the culture medium from the cell culture flask and wash the cells 2x with Hank's solution. Add 1 mL of trypsin per 10 cm² of cells, gently shake to detach the cells, and use a pipette to ensure complete detachment. Incubate at room temperature for 5 min to allow complete lysis, then pipette up and down 7x-10x to fully disrupt the cells. Transfer the lysate to 1.5 mL tubes, centrifuge, and store the supernatant.
- Add an equal volume of chloroform to the supernatant, mix well, and let stand at room temperature for 10 min. Centrifuge at 4 °C for 5 min at 1,000 x g to separate the phases. Carefully transfer the upper aqueous phase to a new tube (if extracting DNA and proteins, retain the lower phase and store it at 4 °C).
- Add isopropanol (0.5 mL per 1 mL of trizol), mix well, and let stand at room temperature for 10 min. Centrifuge at 4 °C for 10 min at 1,000 x g, gently remove the supernatant and discard it.
- Wash the RNA pellet with 75% ethanol, centrifuge at 4 °C for 5 min at 8,000 x g, gently remove the supernatant, and air-dry or vacuum-dry the pellet. Dissolve the RNA in 50 µL of DEPC water, aliquot, and store at -70 °C or proceed to reverse transcription to cDNA.
- Reverse transcription to cDNA
- Perform reverse transcription of cDNA using a kit following manufacturer's instructions.
- Real-time quantitative PCR
- Design primers based on gene sequences and check them using BLAST on the NCBI website. Prepare 10 µM stock solutions of forward and reverse primers for CK20 and β-actin. Table 4 shows the primer sequences.
- Real-Time quantitative PCR
- Add 1 µL of cDNA, 10 µL of 2x master mix, 0.4 µL of forward and reverse primer each, and 8.2 µL of RNase-free water in the reaction system.
- Perform PCR with the following program: Initial denaturation at 95 °C for 2 min, followed by 40 cycles of 95 °C for 15 s and 60 °C for 1 min. Use β-actin as the internal control. Calculate relative expression using the 2-ΔΔCt method.
8. Statistical analysis
- Use a commercial software for statistical analysis. Present data as mean ± standard deviation (mean ± SD). Perform One-Way ANOVA to compare differences among different groups. Define a significant difference as p < 0.05.
Results
Munziq mitigates the pathological changes induced by ischemia-reperfusion injury
To examine the morphological alterations in myocardial tissues, we initially conducted HE staining. As the representative HE staining shown in Figure 1, we observed granular degeneration and vacuolar degeneration in certain myocardial cells in the sham group. Additionally, there was a limited presence of red blood cells and lymphocyte infiltration among myocardial cells. Periodically, we observed vascular dilation and congestion. Myocardial tissues from the MIRI group exhibited more severe impairment and manifested more pronounced morphological changes. These changes included extensive granular degeneration, vacuolar degeneration, red blood cell and lymphocyte infiltration, vascular dilation, and congestion. The myocardial tissue injury in the ABF MIRI group was more severe compared to that observed in the control MIRI group. However, in both the Munziq group, some myocardial cells displayed mild granular degeneration and vacuolar degeneration. The presence of red blood cells and lymphocyte infiltration, vascular dilation, and congestion were scarce. Furthermore, the findings indicate that Munziq exhibits superior cardioprotective effects in ABF MIRI rats and control MIRI rats. In conclusion, these findings suggest that Munziq pretreatment has the potential to mitigate the pathological changes induced by ischemia-reperfusion injury in the heart.
Munziq improved the disruption of mitochondria and cardiac muscle fibers induced by ischemia/reperfusion injury
To further verify the protective role of Munziq in MIRI injury, we examined mitochondrial ultrastructure and cardiac muscle fibers using TEM. As depicted in Figure 2, the myocardial cells in the sham group exhibited an intact structure with closely arranged myofibrils and similar sarcomere length. The myofilament structure appeared clear and slightly loose, while a large number of mitochondria were observed. Conversely, the MIRI group displayed pathological characteristics indicative of damaged myocardial cells, including cell swelling, varied sarcomere length, unclear and loosely arranged myofilament structure. It was noted that there was extensive disruption and dissolution of mitochondria in the I/R group compared with the sham group. Besides, cardiac muscle fibers were irregular and fractured in response to I/R injury. Interestingly, the severity of these changes was notably higher in the ABF MIRI group. In contrast, both groups treated with Munziq exhibited alleviated pathological features associated with damaged myocardial cells. The swelling of myocardial cells was reduced, and the structures of myofibrils, sarcomere, myofilament, and the number of mitochondria were similar to those observed in the sham group. Collectively, these data indicated that Munziq pretreatment could mitigate the disruption of mitochondria and cardiac muscle fibers induced by I/R surgery.
Myocardial ischemia-reperfusion injury exacerbated in ABF MIRI rat and Munziq pretreatment protected cardiac function
The serum levels of cTn-T, CK-MB, and ICAM-1 were detected by using the ELISA method. The results revealed no discernible disparities between the control sham group and the ABF sham group. However, it is noteworthy that the ABF MIRI group exhibited significantly elevated levels of cTn-T (Figure 3A), CK-MB (Figure 3B), and ICAM-1 (Figure 3C) in comparison to the control MIRI group. In order to assess the cardioprotective effects of Munziq, both the control MIRI rats, and the ABF MIRI rats were subjected to Munziq pretreatment. As demonstrated in Figure 3, the levels of cTn-T, CK-MB, and ICAM-1 were notably reduced in the Munziq pretreatment group. These results suggest that Munziq exhibits remarkable cardioprotective properties in ABF MIRI rats.
To investigate the effect of Munziq pretreatment on ischemia reperfusion-induced oxidative stress injury in ABF and control rats, the changes of LDH, MDA, and NO were measured in myocardial tissue. As shown in Figure 3D,F, the ABF MIRI group exhibited significantly elevated levels of MDA and significantly decreased levels of NO in comparison to the control MIRI group. Munziq pretreatment significantly decreased the content of LDH and MDA in ischemic myocardium. At the same time, Munziq pretreatment further increased the level of NO in myocardial tissue.
Pretreatment with Munziq mitigated inflammation of the MIRI by hindering pro-inflammatory cytokines production
Pro-inflammatory cytokines play a critical role in mediating the innate immune response, and pro-inflammatory cytokines were activated during MIRI31. To further investigate the anti-inflammatory effect of Munziq, both the control MIRI rats and ABF MIRI rats underwent Munziq pretreatment. We employed the ELISA method to measure the levels of serum IL-1β, IL-6, and TNF-α and qRT-PCR analysis to evaluate the mRNA levels of IL-1β, IL-6, and TNF-α in myocardial tissue. The results revealed no significant differences in these cytokine levels between the control sham group and the ABF sham group. The levels of IL-1β, IL-6, and TNF-α were significantly upregulated in the ABF MIRI group compared to the control MIRI group. Specifically, IL-1β exhibited a significant increase at both mRNA and protein levels, with a statistically significant difference observed. While there was no difference in mRNA level for IL-6, a disparity was found at the protein level. Similarly, TNF-α did not show any variation in mRNA levels but displayed differences at the protein level. As illustrated in Figure 4, the levels of IL-1β, IL-6, and TNF-α were notably decreased in the Munziq pretreatment group.
Pretreatment with Munziq mitigated the inflammation through the NF-κB signal pathway
The production of cytokines is tightly connected with the activation of NF-κB signaling pathways in most stages of inflammatory response. NF-κB is considered as a potential therapeutic target for inflammatory ailments32. To assess whether the NF-κB pathway is implicated in the anti-inflammation effects of Munziq, we initially conducted Western Blot analysis to measure the expression levels of key constituents of the NF-κB pathway, including NIK, IKKα, pIKKα, and p6513. As depicted in Figure 5, ischemic and reperfusion injury induced notable upregulation of NIK, p-IKKα, and p-p65 within the MIRI group. Treatment with Munziq effectively attenuated this upregulation. The results indicated that Munziq plays a critical role in anti-inflammation by suppressing the activation of the NF-κB signaling pathway.
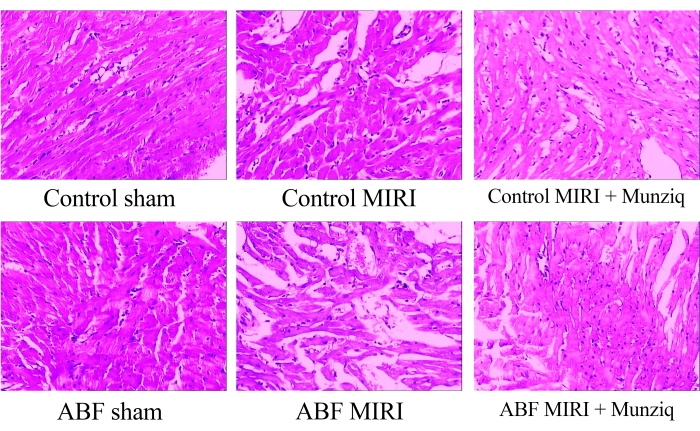
Figure 1: Morphological changes of myocardial tissues observed by HE staining. Representative HE staining images of different groups. Magnification: 100x. Abbreviations: ABF = abnormal body fluid; MIRI = Myocardial Ischemia-reperfusion injury; (n=6). Please click here to view a larger version of this figure.
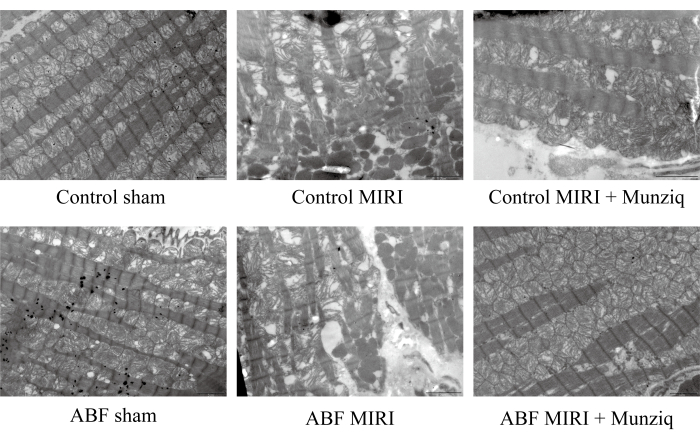
Figure 2: Munziq improved the disruption of mitochondria and cardiac muscle fibers induced by I/R injury. Scanning electron microscope was performed to observe the mitochondria ultrastructure and cardiac muscle fibers changes. Representative images were shown. Scale bar: 5 µm; (n=3). Please click here to view a larger version of this figure.
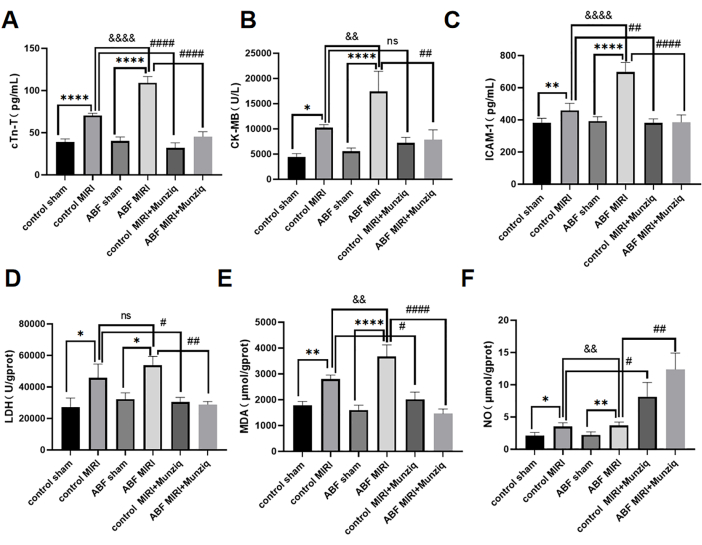
Figure 3: Myocardial ischemia-reperfusion injury exacerbated in ABF MIRI rats, and Munziq pretreatment could protect cardiac function. (A) Serum cTn-T, (B) CK-MB, and (C) ICAM-1. Levels were detected by ELISA, and the changes in LDH, MDA, and NO were measured in myocardial tissue (n=6). *p<0.05, ** p<0.01, ***p<0.001, ****p<0.0001 Compared with sham group; #p<0.05, ## p<0.01, ###p<0.001, ####p<0.0001 Compared with MIRI group; &p<0.05, && p<0.01, &&&p<0.001, &&&&p<0.0001 Compared with control group. Data are presented as mean ± SD. Statistical analysis was performed using one-way ANOVA. Please click here to view a larger version of this figure.
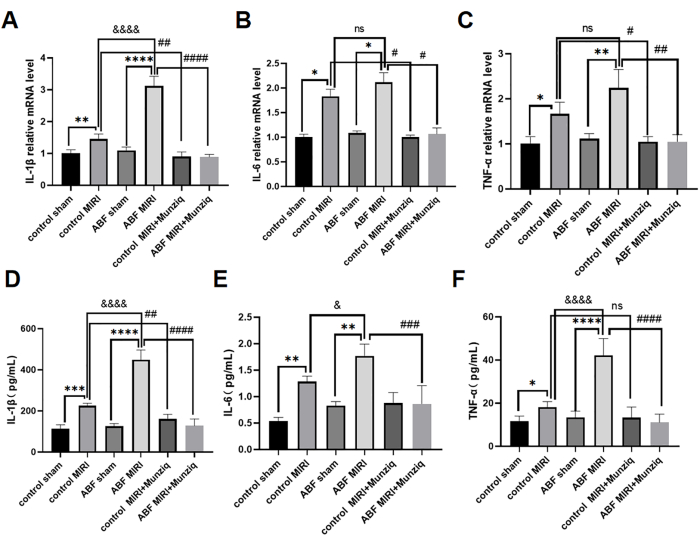
Figure 4: Pretreatment with Munziq mitigated inflammation of the MIRI by preventing the production of pro-inflammatory cytokines. qRT-PCR detected the mRNA levels of (A) IL-1β, (B) IL-6, and (C) TNF-α in myocardial tissue. Measure the (D) IL-1β, (E) IL-6, and (F) TNF-α levels in serum by ELISA (n=6). *p<0.05, ** p<0.01, ***p<0.001, ****p<0.0001 Compared with sham group; #p<0.05, ## p<0.01, ###p<0.001, ####p<0.0001 Compared with MIRI group; &p<0.05, && p<0.01, &&&p<0.001, &&&&p<0.0001 Compared with control group. Data are presented as mean ± SD. Statistical analysis was performed using one-way ANOVA. Please click here to view a larger version of this figure.
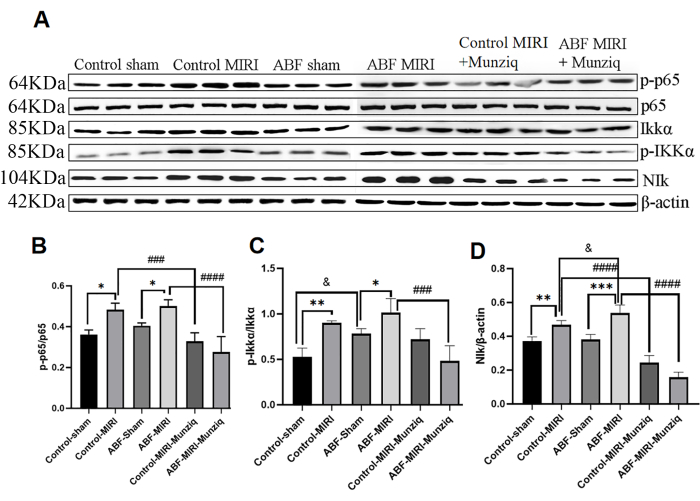
Figure 5: Pretreatment with Munziq mitigated the inflammation through the NF-κB signal pathway. The p-IKKα, IKKα, p-p65, p65, and NIk protein expression in myocardial tissue was detected by Western blot (N = 3 for each group). (A) The protein bands observed in the Western blot analysis. (B-D) The relative expression level is determined based on the gray value. *p<0.05, ** p<0.01, ***p<0.001, ****p<0.0001 Compared with sham group; #p<0.05, ## p<0.01, ###p<0.001, ####p<0.0001 Compared with MIRI group; &p<0.05, && p<0.01, &&&p<0.001, &&&&p<0.0001 Compared with control group. Data are presented as mean ± SD. Statistical analysis was performed using one-way ANOVA. Please click here to view a larger version of this figure.
| Tube Number | Diluent Volume (mL) | BSA Volume (Source) | Final BSA Concentration (mg/mL) |
| A | 0 | 20 (stock solution) | 500 |
| B | 2 | 18 (stock solution) | 400 |
| C | 4 | 16 (stock solution) | 300 |
| D | 6 | 14 (stock solution) | 200 |
| E | 8 | 12 (stock solution) | 150 |
| F | 12 | 8 (stock solution) | 100 |
| G | 16 | 4 (stock solution) | 50 |
| H | 20 | 0 (stock solution) | 0 |
Table 1: BSA standard concentration preparation chart.
| Separating Gels | 15% Stacking Gel | 12% Stacking Gel | 8% Stacking Gel | 5% Stacking Gel | |
| Reagents | Volume | Volume | Volume | Reagents | Volume |
| Deionized water (mL) | 2.76 | 3.96 | 5.52 | Deionized water (mL) | 4 |
| 30% Acrylamide (mL) | 6 | 4.8 | 3.24 | 30% Acrylamide (mL) | 1 |
| 1.5mol/lTris.HCl(PH8.8)(mL) | 3 | 3 | 3 | 1.0M Tris.HCl (pH6.8; mL) | 1 |
| 10%SDS(μL) | 120 | 120 | 120 | 10%SDS(μL) | 80 |
| 10%AP(μL) | 120 | 120 | 120 | 10%AP(μL) | 60 |
| TEMED(μL) | 4.8 | 4.8 | 7.2 | TEMED(μL) | 8 |
| Total Volume (mL) | 12 | 12 | 12 | Total Volume (mL) | 6 |
Table 2: Gel concentration preparation.
| Primary Antibody | Dilution Ratio | Secondary Antibody | Dilution Ratio |
| β-actin | 1:1000 | Goat anti-Mouse IgG H&L (HRP) | 1:15000 |
| p65 | 1:1000 | Goat anti-Rabbit IgG H&L (HRP) | 1:5000 |
| p-p65 | 1:300 | 1:5000 | |
| BCL-2 | 1:1000 | 1:5000 | |
| BAX | 1:1000 | 1:5000 | |
| Drp1 | 1:800 | 1:5000 | |
| Fis1 | 1:1000 | 1:5000 | |
| Mfn2 | 1:800 | 1:5000 |
Table 3: Antibody dilution ratios.
| Gene | Primer sequence (5' to 3') | ||
| IL-1β-F | CTGTGACTCGTGGGATGATG | ||
| IL-1β-R | GGGATTTTGTCGTTGCTTGT | ||
| TNF-α-F | GCCTCCTCTCTGCCATCAAG | ||
| TNF-α-R | CTCCAAAGTAGACCTGCCCG | ||
| IL-6-F | GCCCACCAGGAACGAAAGTC | ||
| IL-6-R | GGCAACTGGCTGGAAGTCTC | ||
| beta actin-F | CCCATCTATGAGGGTTACGC | ||
| beta actin-R | TTTAATGTCACGCACGATTTC | ||
Table 4: Primer sequences.
Discussion
MIRI, which stands for myocardial ischemia-reperfusion injury, is a common and significant complication that occurs after myocardial reperfusion in cardiac surgery36,37, few drugs or adjuvants have definitively improved clinical outcomes compared to contemporaneous controls38. Therefore, there is an urgent need for effective therapies to protect the heart from such injury. Munziq, a traditional herbal preparation, has demonstrated certain therapeutic effects on heart ischemia-reperfusion injury27,28,30. However, these findings are preliminary, and the underlying mechanisms remain unknown. The purpose of this study was to further investigate and validate Munziq's protective effects and mechanisms on MIRI.
Abnormal body fluid is referred to as a common manifestation of various chronic illnesses, including cardiovascular diseases, according to traditional Uyghur medicine39,40. In the Uyghur medical system, ABF is believed to be a result of long-term stress and is referred to as the source of various diseases39. In this study, an abnormal body fluid model was employed to simulate the underlying characteristics of cardiovascular diseases that necessitate cardiac surgery. MIRI was induced in rats with ABF to determine the myocardial ischemia-reperfusion injury.
Ischemia reperfusion injury is a complex pathological condition that involves multiple processes. It has been reported that ischemia-reperfusion in the heart leads to pathological alterations in the myocardium4. These pathological changes primarily manifest as shortened cardiomyocytes, disrupted sarcomeric structure, mitochondrial swelling41, and loosely arranged sarcomeric myofibrils, as observed in this study. Myocardial injury also results in alterations in serum enzymes. It is well-known that levels of cTnT, CK-MB, and ICAM-1 in the serum increase following myocardial injury42,43. Therefore, these biomarkers are used to assess the extent of acute myocardial injury. In this study, we observed the pathological changes in the myocardium and the elevation of serum enzymes after ischemia-reperfusion injury, indicating the successful establishment of the MIRI model. The results showed that myocardial injury in the ABF MIRI group was more severe compared to the control MIRI group. Following treatment with Munziq, the pathological changes were noticeably alleviated, and serum enzyme levels significantly decreased. Collectively, these findings demonstrate the cardio-protective effects of Munziq during MIRI.
In this study, the left anterior descending (LAD) artery was ligated for 30 min, followed by reperfusion for 120 min to establish an ischemia-reperfusion injury model in rats. A meta-analysis44 summarizing 43 studies used to create ischemia-reperfusion injury models in rats indicated that ischemia times ranged from 30 to 60 min, with reperfusion duration of 30 to 120 min. Some studies adopted a protocol of 5 min of ischemia followed by 5 min of reperfusion, repeated 4x45. Among these methods, a protocol involving 30 min of ischemia and 120 min of reperfusion was utilized most frequently, appearing in 19 cases. During the experimental process, we observed a pale coloration of the myocardium through direct visual inspection following 30 min of ischemia. In addition to visual assessment of myocardial color changes, electrocardiogram (ECG) monitoring was employed to identify signs of ischemia, such as ST-segment elevation. Serum markers, including troponin and creatine kinase, as well as hemodynamic parameters like heart rate (HR), left ventricular diastolic pressure (LVDP) and left ventricular systolic pressure (LVSP), can all serve as indicators to evaluate the effectiveness of the ischemia model46. In this study, we primarily relied on visual observation to assess the effects of ischemia and reperfusion. Due to individual variations among rats, it is advisable to supplement visual inspection with additional objective measures to assess the efficacy of ischemia rather than applying a uniform ischemic duration to all rats.
In mammals, the NF-κB family consists of five members, one of which is p6513,47. There are two main pathways of activation of NF-κB in cells47. In the canonical pathway, activation of the IKK complex (IKKα, IKKβ, and IKKγ) leads to the phosphorylation of IκB proteins, initiating NF-κB activation. In the noncanonical NF-κB pathway, NF-κB activation is mediated by the phosphorylation of NIK and IKKα. Extensive evidence suggests that the NF-κB pathway plays a crucial role in mediating ischemia and reperfusion injury48,49,50. Interfering with NF-κB activation can attenuate injury induced by ischemia and reperfusion51,52. Interestingly, there was more activation of the NF-κB signaling pathway in the ABF MIRI group and higher levels of downstream inflammatory cytokines.
Numerous traditional Chinese medicine drugs have been shown to exert protective effects against ischemia and reperfusion injury by modulating the NF-κB pathway. For instance, Liu et al.53 found that quercetin mitigated MIRI by inhibiting the NF-κB pathway. Han et al.54 reported that hydroxysafflor yellow A alleviated MIRI by inhibiting TLR4/NF-κB signaling. However, whether Munziq exerts a protective role in MIRI through the NF-κB signaling pathway remains unexplored. To further elucidate the mechanism of Munziq, this study analyzed the expression levels of key nodes in the NF-κB signaling pathway, including NIK, IKKα, pIKKα, and p65. The results demonstrated that the expression levels of these proteins were significantly downregulated following Munziq treatment, indicating that Munziq inhibits the expression of key nodes in the NF-κB signaling pathway during MIRI.
There is extensive evidence supporting the notion that the activation of NF-κB triggers the production of inflammatory proteins and adhesion molecules, resulting in the recruitment of lymphocytes55. For instance, Valen et al.56 discovered that NF-κB was activated and that levels of IL-1β and TNF-α were elevated during myocardial ischemia/reperfusion. Furthermore, IL-6, a pro-inflammatory cytokine regulated by NF-κB, exhibits increased expression during reperfusion57. Consistent with these findings, the results of this study demonstrate that Munziq significantly inhibits the levels of proinflammatory cytokines (including IL-1β, IL-6, and TNF-α) in the context of myocardial ischemia/reperfusion injury (MIRI). From this, it can be inferred that Munziq exerts its inhibitory effects on the expression of proinflammatory cytokines through the suppression of the NF-κB signaling pathway.
In conclusion, the results suggest that MIRI was more serious in ABF. Munziq has cardioprotective effects in ischemia and reperfusion injury. This protective effect may be acted by suppressing the NF-κB signaling pathway. These findings suggest that Munziq holds great potential as a therapeutic agent for safeguarding the heart against reperfusion injury during cardiac surgery.
There are several limitations of the study. Firstly, the study is conducted in a rat model, which, despite being a valuable translational model, may not fully replicate the complexity of human cardiovascular condition. Secondly, the study primarily focuses on the NF-κB signaling pathway as a mechanistic underpinning of Munziq's effects. However, MIRI is a multifactorial process that may involve various other signaling pathways and molecular mechanisms.
While the current study provides valuable insights into the potential cardioprotective effects of Munziq in a rat model of myocardial ischemia-reperfusion injury (MIRI), particularly in the context of abnormal body fluid (ABF), there are several areas for future exploration. One avenue for further research is to conduct in vitro studies using cultured cardiomyocytes to directly observe the effects of Munziq on cellular responses to ischemia and reperfusion. Additionally, adopting a multi-omics approach could offer a more comprehensive view of the molecular changes associated with Munziq treatment, potentially revealing additional pathways and mechanisms that contribute to its cardioprotective effects. It would also be beneficial to explore different doses of Munziq to determine the optimal dosage for maximal cardioprotection while minimizing potential side effects. Long-term studies are necessary to assess the sustained effects of Munziq on cardiac function and to monitor for any potential adverse effects associated with prolonged use.
Disclosures
The authors have nothing to disclose.
AUTHOR CONTRIBUTION:
Duolikun Mutailifu performed the experiments, Abudusaimi Aini wrote the initial draft of the manuscript and analyzed the data; Aili Aibibula contributed to the conception and design of the study; Zheng Liu and Abudunaibi Maimaitiaili participated in the design of the study; Abudunaibi Maimaitiaili arranged the study funds; and all authors read and approved the final manuscript.
Acknowledgements
This work was supported by the National Natural Science Foundation of China [grant Number: 82060907] and the 'Tianshan Elite' High-Level Medical and Health Talent Cultivation Program [Grant No. TSYC202301B004].
Materials
| Name | Company | Catalog Number | Comments |
| ABI 7500 Real-time PCR | ABI, CA, USA | Used for performing qRT-PCR. | |
| Adult male Sprague-Dawley (SD) rat | Animal Experimental Center of Xinjiang Medical University | ||
| Anti-NF-kB p65, anti-NF-kB Inducing Kinase NIK, anti-IKK alpha, anti-IKK alpha (phospho T23), anti-β-actin | Abcam, CA, USA | Used for Western blot analysis targeting specific proteins. | |
| Anti-TTC11/FIS1 Antibody | abcam | ab71498 | Used for protein detection in Western Blot (WB) experiments. |
| BCA Protein Assay Kit | Tiangen Biotech Co., Ltd., Beijing, China | Used for determining protein concentration. | |
| beta-Actin Loading Control antibody Mouse Mab | Sino Biological | 100166-MM10 | Used for protein detection in Western Blot (WB) experiments. |
| ChemiScope 3300 Imaging System | Clinx Science Instruments, Shanghai, China | Used for scanning developed films from Western blot analysis. | |
| ELISA kit for CK-MB detection | Nanjing Jiancheng Bioengeering Institute(Nanjing, China) | Used for detecting levels of cardiac injury indexes | |
| ELISA kit for cTn-T, ICAM-1, IL-1β, IL-6, TNF-α detection | CUSABIO Biotech CO., Ltd. (Wuhan, China) | Used for detecting levels of cardiac injury indexes and inflammatory cytokines in rat serum samples. | |
| FastQuant RT Kit | TIANGEN, Beijing China | Used for reverse transcription of cDNA. | |
| HRP conjugated goat anti-mouse IgG, HRP conjugated goat anti-rabbit IgG | Thermo Scientific, Basingstoke, UK | ||
| LDH Assay kit and MDA Assay kit | Jiancheng Biotech Co., Ltd, Nanjing, China | Used for detecting LDH and MDA levels in myocardium tissues from the infarct area in the left ventricle. | |
| Munziq | provided by Xinjiang Medical University | The main investigational drug in this study | |
| NF-κB p65 (D14E12) XP Rabbit mAb #8242 | CST | 8242S | Used for protein detection in Western Blot (WB) experiments. |
| Nitric Oxide (NO) assay kit | Jiancheng Biotech Co., Ltd, Nanjing, China | Used for detecting NO levels at 550 nm in myocardial tissue. | |
| Phospho-NF-κB p65 (Ser536) (93H1) Rabbit mAb #3033 | CST | 3033S | Used for protein detection in Western Blot (WB) experiments. |
| Quantity One software | Bio-Rad Laboratories, Hercules, CA, USA | Used for analyzing Western blot images. | |
| Recombinant Anti-DRP1 Antibody | abcam | ab184247 | Used for protein detection in Western Blot (WB) experiments. |
| RIPA lysis buffer | Boster Biotechnology Co., Ltd., Wuhan, China | AR0105 | Used for extracting total proteins from rat ventricular tissues. |
| Secondary antibodies (HRP conjugated goat anti-mouse IgG, HRP conjugated goat anti-rabbit IgG) | Thermo Scientific, Basingstoke, UK | Used for detection following primary antibody incubation in Western blot analysis. | |
| SYBR Select Master Mix | ABI, CA, USA | Used in qRT-PCR analysis on ABI 7500 Real-time PCR instrument. | |
| transmission electron microscope | HitachiS-2400 Hitachi, Tokyo, Japan | Used to examine thin sections of ventricular tissues. | |
| Trizol reagent | Invitrogen Co., Carlsbad, California, USA | Used for RNA extraction from ventricular tissues. |
References
- Anderson, J. L., Campion, E. W., Morrow, D. A. Acute myocardial infarction. New Engl J Med. 376 (21), 2053-2064 (2017).
- Vos, T., et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: A systematic analysis for the global burden of disease study 2019. Lancet. 396 (10258), 1204-1222 (2020).
- Murphy, A., Goldberg, S. Mechanical complications of myocardial infarction. Am J Med. 135 (12), 1401-1409 (2022).
- Heusch, G. Myocardial ischemia/reperfusion: Translational pathophysiology of ischemic heart disease. Medicine. 5 (1), 10-31 (2024).
- Bhatt, D. L., Lopes, R. D., Harrington, R. A. Diagnosis and treatment of acute coronary syndromes. Jama. 327 (7), 662-675 (2022).
- Hausenloy, D. J., et al. The coronary circulation in acute myocardial ischaemia/reperfusion injury: A target for cardioprotection. Cardiovasc Res. 115 (7), 1143-1155 (2019).
- Gunata, M., Parlakpinar, H. A review of myocardial ischaemia/reperfusion injury: Pathophysiology, experimental models, biomarkers, genetics and pharmacological treatment. Cell Biochem Funct. 39 (2), 190-217 (2020).
- He, J., et al. Myocardial ischemia/reperfusion injury: Mechanisms of injury and implications for management (review). Exp Ther Med. 23 (6), 430 (2022).
- Xiang, M., et al. Role of oxidative stress in reperfusion following myocardial ischemia and its treatments. Oxid Med Cell Longevity. 2021, 1-23 (2021).
- Astudillo, A. M., Balboa, M. A., Balsinde, J. Compartmentalized regulation of lipid signaling in oxidative stress and inflammation: Plasmalogens, oxidized lipids and ferroptosis as new paradigms of bioactive lipid research. Prog Lipid Res. 89, 101207 (2023).
- Algoet, M., et al. Myocardial ischemia-reperfusion injury and the influence of inflammation. Trends Cardiovasc Med. 33 (6), 357-366 (2023).
- Zhang, F., et al. Β-cryptoxanthin alleviates myocardial ischaemia/reperfusion injury by inhibiting nf-κb-mediated inflammatory signalling in rats. Arch Physiol Biochem. 128 (4), 1128-1135 (2020).
- Guo, Q., et al. Nf-κb in biology and targeted therapy: New insights and translational implications. Signal Transduct Target Ther. 9 (1), 53 (2024).
- Mi, J., et al. Inhibition of heat shock protein family a member 8 attenuates spinal cord ischemia-reperfusion injury via astrocyte nf-κb/nlrp3 inflammasome pathway. J Neuroinflammation. 18 (1), 170 (2021).
- Zhang, L., Zhao, S., Wang, Y. Diannexin alleviates myocardial ischemia-reperfusion injury by orchestrating cardiomyocyte oxidative damage, macrophage polarization and fibrotic process by tlr4-nf-kb-mediated inactivation of nlrp3 inflammasome. Int Immunopharmacol. 130, 111668 (2024).
- Zhou, Y., et al. Qingchang mixture prevents the intestinal ischemia-reperfusion injury through tlr4/nf-kb pathway. Combinatorial Chem High Throughput Screen. 26 (1), 49-57 (2023).
- Ozturk, A., et al. The protective effects of trimetazidine against ovary ischemia-reperfusion injury via the tlr4/nf-kb signal pathway. J Biochem Mol Toxicol. 36 (8), e23114 (2022).
- Du, S., Deng, Y., Yuan, H., Sun, Y. Safflower yellow b protects brain against cerebral ischemia reperfusion injury through ampk/nf-kb pathway. Evid Based Compl Alt Med. 2019, 1-11 (2019).
- Dong, X., et al. Physcion protects rats against cerebral ischemia-reperfusion injury via inhibition of tlr4/nf-kb signaling pathway. Drug Design Dev Therapy. 15, 277-287 (2021).
- Hashmi, S., et al. Hydrogen sulphide treatment prevents renal ischemia-reperfusion injury by inhibiting the expression of icam-1 and nf-kb concentration in normotensive and hypertensive rats. Biomolecules. 11 (10), 1549 (2021).
- Gao, L., et al. Hic-5 deficiency attenuates hepatic ischemia reperfusion injury through tlr4/nf-κb signaling pathways. Life Sciences. 249, 117517 (2020).
- Xu, Z., et al. Cardioprotection of mab2g4/odn/lip on myocardial ischemia-reperfusion injury via inhibiting the nf-κb signaling pathway. Cardiovas Ther. 2023, 1-15 (2023).
- Xiao, G., et al. Cxcr1 and its downstream nf-κb inflammation signaling pathway as a key target of guanxinning injection for myocardial ischemia/reperfusion injury. Front Immunol. 13, 1007341 (2022).
- Jeddi, S., Gheibi, S., Kashfi, K., Carlström, M., Ghasemi, A. Dose-dependent effects of long-term administration of hydrogen sulfide on myocardial ischemia-reperfusion injury in male wistar rats: Modulation of rkip, nf-κb, and oxidative stress. Int J Mol Sci. 21 (4), 1415 (2020).
- Yao, Y., et al. Targeting camkii-δ9 ameliorates cardiac ischemia/reperfusion injury by inhibiting myocardial inflammation. Circ Res. 130 (6), 887-903 (2022).
- Zhuang, L., Zong, X., Yang, Q., Fan, Q., Tao, R. Interleukin-34-nf-κb signaling aggravates myocardial ischemic/reperfusion injury by facilitating macrophage recruitment and polarization. eBioMedicine. 95, 104744 (2023).
- Maimaitiaili, A., et al. Effects of different doses of savda munziq on myocardial ischemia-reperfusion injury in rats with abnormal savda syndrome. Genet Mol Res. 13 (3), 4729-4735 (2014).
- Abudunaibi, M., et al. Myocardial protective effects of munziq in myocardial ischemia-reperfusion injury rats with abnormal savda syndrome. Genet Mol Res. 14 (2), 3426-3435 (2015).
- Hao, Y., et al. Transition of the abnormal savda syndrome to the hepatic carcinoma shifted unfolded protein response to autophagy was partly reversed by savda munziq in a rat model. Biomed Pharmacother. 121, 109643 (2020).
- Maimaitiaili, A., Li, J., Aibibula, A., Abudureheman, M. Erratum: Inhibition of nuclear factor kappa b pathway protects myocardial ischemia/reperfusion injury in rats under treatment with fufang munziq granule (munziq). Am J Transl Res. 10 (11), 3876 (2018).
- Zhang, X., et al. Rosa rugosa flavonoids alleviate myocardial ischemia reperfusion injury in mice by suppressing jnk and p38 mapk. Microcirculation. 24 (7), (2017).
- Dong, P., Liu, K., Han, H. The role of nf-κb in myocardial ischemia/reperfusion injury. Curr Prot Peptide Sci. 23 (8), 535-547 (2022).
- Guo, X., et al. Differential integrative omic analysis for mechanism insights and biomarker discovery of abnormal savda syndrome and its unique munziq prescription. Sci Rep. 6, 27831 (2016).
- Awad, A. S. Role of at1 receptors in permeability of the blood-brain barrier in diabetic hypertensive rats. Vascul Pharmacol. 45 (3), 141-147 (2006).
- La, X., et al. Upregulation of pd-1 on cd4(+)cd25(+) t cells is associated with immunosuppression in liver of mice infected with echinococcus multilocularis. Int Immunopharmacol. 26 (2), 357-366 (2015).
- Wu, T., et al. Circulating small extracellular vesicle-encapsulated sema5a-it1 attenuates myocardial ischemia-reperfusion injury after cardiac surgery with cardiopulmonary bypass. Cell Mol Biol Lett. 27 (1), 95 (2022).
- Pinto, A., et al. The extracellular isoform of superoxide dismutase has a significant impact on cardiovascular ischaemia and reperfusion injury during cardiopulmonary bypass. Eur J Cardio-Thor Surg. 50 (6), 1035-1044 (2016).
- Sabe, S. A., Harris, D. D., Broadwin, M., Sellke, F. W. Cardioprotection in cardiovascular surgery. Basic Res Cardiol. 119 (4), 545-568 (2024).
- Abudunaibi, M., et al. Myocardial protective effects of munziq in myocardial ischemia-reperfusion injury rats with abnormal savda syndrome. Genet Mol Res. 14 (2), 3426-3435 (2015).
- Mamtimin, B., et al. An magnetic resonance-based plasma metabonomic investigation on abnormal savda in different complicated diseases. J Tradl Chinese Med. 34 (2), 166-172 (2014).
- Marin, W., Marin, D., Ao, X., Liu, Y. Mitochondria as a therapeutic target for cardiac ischemia-reperfusion injury (review). Int J Mol Med. 47 (2), 485-499 (2020).
- Novack, V., et al. Troponin criteria for myocardial infarction after percutaneous coronary intervention. Arch Intern Med. 172 (6), 502-508 (2012).
- Kemp, M., Donovan, J., Higham, H., Hooper, J. Biochemical markers of myocardial injury. Br J Anaesth. 93 (1), 63-73 (2004).
- Zhang, D. Z., Jia, M. Y., Wei, H. Y., Yao, M., Jiang, L. H. Systematic review and meta-analysis of the interventional effects of resveratrol in a rat model of myocardial ischemia-reperfusion injury. Front Pharmacol. 15, 1301502 (2024).
- Ta, F. X., Zhang, T., Zhu, C. M. Correlation between mir-21 and the protective effects of resveratrol against myocardial ischemia/reperfusion injury in rats. Chin. J. Arteriosclerosis. 21 (6), 493-496 (2013).
- Lindsey, M. L., et al. Guidelines for experimental models of myocardial ischemia and infarction. Am J Physiol Heart Circ Physiol. 314 (4), H812-H838 (2018).
- Oeckinghaus, A., Hayden, M. S., Ghosh, S. Crosstalk in nf-kappab signaling pathways. Nat Immunol. 12 (8), 695-708 (2011).
- Van Der Heiden, K., Cuhlmann, S., Luong Le, A., Zakkar, M., Evans, M. Role of nuclear factor kappab in cardiovascular health and disease. Clin Sci. 118 (10), 593-605 (2010).
- Ha, T., et al. Toll-like receptors: New players in myocardial ischemia/reperfusion injury. Antioxid Redox Signal. 15 (7), 1875-1893 (2011).
- Pourrajab, F., Yazdi, M. B., Zarch, M. B., Zarch, M. B., Hekmatimoghaddam, S. Cross talk of the first-line defense tlrs with pi3k/akt pathway, in preconditioning therapeutic approach. Mol Cell Ther. 3, 4 (2015).
- Wang, Y. H., et al. Lumbrokinase attenuates myocardial ischemia-reperfusion injury by inhibiting tlr4 signaling. J Mol Cell Cardiol. 99, 113-122 (2016).
- Yu, H., et al. Gypenoside protects cardiomyocytes against ischemia-reperfusion injury via the inhibition of mitogen-activated protein kinase mediated nuclear factor kappa b pathway in vitro and in vivo. Front Pharmacol. 7, 148 (2016).
- Liu, X., et al. Peroxisome proliferator-activated receptor gamma (ppargamma) mediates the protective effect of quercetin against myocardial ischemia-reperfusion injury via suppressing the nf-kappab pathway. Am J Transl Res. 8 (12), 5169-5186 (2016).
- Han, D., et al. Hydroxysafflor yellow a alleviates myocardial ischemia/reperfusion in hyperlipidemic animals through the suppression of tlr4 signaling. Sci Rep. 6, 35319 (2016).
- Yang, Q., He, G. W., Underwood, M. J., Yu, C. M. Cellular and molecular mechanisms of endothelial ischemia/reperfusion injury: Perspectives and implications for postischemic myocardial protection. Am J Transl Res. 8 (2), 765-777 (2016).
- Valen, G., Paulsson, G., Vaage, J. Induction of inflammatory mediators during reperfusion of the human heart. Ann Thorac Surg. 71 (1), 226-232 (2001).
- Saini, H. K., et al. Role of tumour necrosis factor-alpha and other cytokines in ischemia-reperfusion-induced injury in the heart. Exp Clin Cardiol. 10 (4), 213-222 (2005).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved