A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Enhanced Reproducibility and Precision of High-Throughput Quantification of Bacterial Growth Data Using a Microplate Reader
In This Article
Summary
Here, a high-throughput protocol is presented to measure growth data, including growth curves, growth rate, and maximum growth rate. The protocol was verified and validated using two biofilm-producing bacteria. The results and approach applied in this study can be expanded to other high-throughput protocols using microplate readers.
Abstract
This study aimed to develop a repeatable, reliable, high-throughput protocol to monitor bacterial growth in 96-well plates and analyze the maximum growth rate. The growth curves and maximum growth rates of two bacterial species were determined. Issues including (i) lid condensation, (ii) pathlength correction, (iii) inoculation size, (iv) sampling time interval, and (v) spatial bias were investigated. The repeatability of the protocol was assessed with three independent technical replications, with a standard deviation of 0.03 between the runs. The maximum growth rates of Bacillus mycoides and Paenibacillus tundrae were determined to be (mean ± SD) 0.99 h−1 ± 0.03 h−1 and 0.85 h−1 ± 0.025 h−1, respectively. These bacteria are more challenging to monitor optically due to their affinity to clump together. This study demonstrates the critical importance of inoculation size, path length correction, lid warming, sampling time intervals, and well-plate spatial bias to obtain reliable, accurate, and reproducible data on microplate readers. The developed protocol and its verification steps can be expanded to other methods using microplate readers and high-throughput protocols, reducing the researchers' innate errors and material costs.
Introduction
Developing interest in multi-omics manipulation, including mechanism and metabolic studies of bacteria, emphasizes the importance of high-throughput and automated methods such as recording growth data1,2. Growth data comprising kinetic parameters, such as maximum growth rates, can help characterize bacterial responses to different physical, chemical, and antibacterial conditions. Growth rate data are a standard response variable utilized to uncover potential genotype-phenotypes linkages1 or indicate the microbial safety and shelf life of food produce3,4. Techniques such as adaptive laboratory evolution5,6,7, genome-wide screening, certain chemical assays8, and various forward genetic screens9 rely on growth rates to evaluate the results.
Optical density (OD) measurements of bacterial cultures are a standard microbiological method to monitor bacterial growth. OD measurements are often recorded at a wavelength of 600 nm, relying on light scattering and the cell density10,11. The Beer-Lambert law explains the OD values' dependency on the concentration (i.e., cell density, cell number), path length, and absorptivity coefficient. The geometry and optical system of a spectrophotometer influence the OD readings11. Classical methods of OD measurements can be very time- and labor-intensive, and the data can carry a variety of human errors. In this protocol, a microplate reader is used to decrease the analyst time12,13 and the chance of biological contamination. High-throughput analysis using microplate readers is broadly applied in different microbiology areas, such as screening biofilm-producing bacteria14,15, bacterial growth inhibition16, yeast cell growth17, the determination of antifungal susceptibility18, and toxicity screening of nanomaterials19.
A few researchers have published bacterial growth rate protocols using a microplate reader12,20,21. However, a thorough protocol that examines the reliability of collected data has not been fully established. It is reported that factors such as the type of species22,23,24 and sealing tapes impact the repeatability due to the oxygen transfer inadequacy in a 96-well plate25,26. Delaney et al. reported large clusters of Methylorubrum extorquens (wild-type strain) in the growth medium when using a microplate reader, which caused extremely noisy growth data24. The issue was resolved by removing the genes associated with biofilm production24. Due to the secretion of extracellular polymeric substances, biofilm-producing bacteria have a greater affinity to coalesce together and create cell clusters. Therefore, it is more challenging to monitor their growth using light scattering techniques (e.g., spectrophotometers and microplate readers).
This protocol aims to establish steps to obtain reproducible data in a high-throughput method using a microplate reader. Bacillus mycoides and Paenibacillus tundrae were used due to their fast growth and biofilm-producing ability, which are traditionally challenging in manual and automated approaches. Factors such as (i) pathlength correction, (ii) condensation on the lid, (iii) inoculum size, (iv) sampling time interval, and (v) spatial bias were investigated to assess the reliability and reproducibility of the data. This protocol presents steps for accurately monitoring bacterial growth and measuring specific growth rates using a microplate reader.
Protocol
NOTE: All steps in this protocol must be followed in sterile conditions (i.e., between two flames or a biosafety cabinet). All materials and tools are autoclaved for 20 min. See the Table of Materials for details about all materials, equipment, and software used in this protocol. Gloved hands are disinfected, kept wet with hand disinfectant or 70% alcohol solution for at least 1 min, and not removed from the safety cabinet afterward. Otherwise, the disinfecting procedure must be repeated before introducing hands back into the safety cabinet. CAUTION: Ensure the disinfectant is completely evaporated before using an open flame.
NOTE: Two bacteria were isolated from drinking water biofiltration as explained previously27 for their ability to produce biofilm. They were identified by the full-16S rRNA sequencing and submitted to NCBI as Bacillus mycoides (SAMN10518261) and Paenibacillus tundrae (SAMN10452279).
1. Preparing Bacterial Stock in Glycerol
- Weigh out 3 g of Tryptic soy broth (TSB) powder and dissolve it in 100 mL of distilled water. Autoclave the broth for 20 min and let it cool down to room temperature. Add 50 mL of TSB to a 50 mL flask.
- Transfer the bacteria to agar plates27,28. Using a loop, pick a colony of bacteria from the agar plate. Add the loop's content into the flask by gently swirling the loop. Incubate the flask overnight at 30 °C ± 1 °C with orbital shaking at 150 rpm.
NOTE: Temperature and shaking need to be adjusted according to the strain type. - Prepare another 50 mL flask with 47.5 mL of TSB. Transfer 2.5 mL of the overnight culture to the flask. Incubate it for 5-6 h to reach OD600 = 0.6 0.05 (to get to the mid-exponential phase).
- Transfer the culture to a 50 mL sterilized centrifuge tube. Centrifuge the culture at 2,200 × g for 10 min. Decant the broth gently to avoid losing any cells collected at the bottom of the tube.
- Add 5 mL of phosphate-buffered saline (PBS) to the same tube and centrifuge at 2,200 × g for 10 min. Repeat this step 3x.
- Adjust the OD600 of the cells to 0.6 0.05 by using a pipette to titrate an adequate amount of PBS solution. Accurately record the added amount of PBS for future use.
- Titrate glycerol to the suspension to reach 20% v/v. Cap the tube and vortex for 15 s.
- Transfer 1.5 mL of the suspension to 2 mL cryovials. Put the cryovials in an appropriate box and store them in a freezer at −80 °C.
2. Prepare Overnight Culture.
- Let the cryovials with bacterial stock thaw at room temperature for 30 min or until the content is liquid.
NOTE: It is recommended not to accelerate the thawing process. - Prepare a sterilized tube with 10 mL of TSB. Transfer 50 µL of the stock suspension to the tube. Incubate the tube overnight (16-18 h) at 30 °C ± 1 °C with orbital shaking at 150 rpm.
3. Prepare the Inoculum.
- Prepare a tube with 10 mL of sterilized TSB. Transfer 500 µL of the overnight culture to the tube. Incubate it for 6-8 h to reach OD600 = 0.6 0.05 (or get to the mid-exponential phase).
- Transfer the culture to a 15 mL centrifuge tube. Centrifuge the tube at 2,200 × g for 10 min. Decant the broth gently.
- Add 5 mL of PBS and centrifuge at 2,200 × g for 10 min. Repeat this step 3x.
- Adjust the OD600 of the cells to 0.6 0.05 by titrating PBS solution using a pipette. Accurately record the added amount of PBS for future use.
4. Transferring Growth Medium to the Microplate Reader
- Choose a transparent, sterile, flat-bottom well plate with a lid (i.e., 96-well plate, 6-well plate, 12-well plate).
- Measure the pathlength correction for the microplate using DI water before running the protocol, as explained in step 1.10.
- Draw a reference table based on the selected well plate to specify the samples' positions before loading. For example, draw an 8 x 12 table for a 96-well plate.
NOTE: The reference table avoids confusion during the incubation and result collection steps. - Place the following items in a biosafety cabinet: a microplate with a lid, pipettor 1,000 µL and 200 µL, 1,000 µL and 200 µL pipette tips, several cryovials, a 25 mL beaker, the growth medium, and the inoculum.
NOTE: The mentioned items should be transferred to the biosafety cabinet aseptically. - Add 10 mL of growth medium to the beaker. Transfer the calculated amount of inoculum to the beaker with the growth medium.
NOTE: The inoculum ratios in this protocol are 1% and 5%. For example, to achieve a 5% and 1% inoculum ratio, add 500 µL and 100 µL of the inoculum to 9.5 mL and 9.9 mL of the growth medium, respectively. - After adding the inoculum, gently shake the beaker to get a uniform distribution. Dispense 200 µL of the inoculated medium into the designated wells using the reference table.Choose at least three wells to act as controls by adding 200 µL of the growth medium (with no inoculum).
NOTE: Ensure that the control wells are clearly marked in the reference table. If any growth (contamination) is observed in these wells, the experiment should be repeated. - Dispense 200 µL of DI water into the edge wells where the evaporation rate is higher.Place the lid gently before removing the 96-well plate from the safety cabinet. Place the 96-well plate in the plate reader carefully, ensuring no sudden movements.
5. Microplate Reader Settings
- Turn on the microplate reader and then use the embedded software to create the customized protocol. In Task Manager, open protocol and choose Create New | Standard Protocol. Open Procedure and adjust the settings as follows:
- Set the temperature to 30 °C (or the targeted values) using Set Temperature.
- Click on Incubator On and then set the Gradient to 1 °C.
NOTE: To avoid unreliable data due to condensation on the lid, ensure that Gradient is set to 1 °C. - Choose the Preheat option, ensuring uniform heat distribution along the well plate.
- Open Start Kinetic and choose the desired incubation duration using the Run time (e.g., 12:00:00 or 24:00:00, etc.) and Interval between readings for 00:30:00 (represents 30 min) or 01:00:00 (represents 1 h).
NOTE: Reading each 96-well plate takes approximately 1 min. - Set the Shaking settings as follows: open Shake; set Shake Mode to Linear; click on Continuous Shake; and adjust the Frequency to 567 cpm (or any desired value).
- Set the OD reading settings as follows: click on Read | Absorbance; select Endpoint/Kinetic and Monochromators; set Wavelength to 600 nm; and click Validate | Save to save it as a new protocol with the appropriate name and date.
6. Recording Growth Data
- Open Task Manager | Read Now.
- Choose the saved protocol, then click on OK to begin the protocol.
- Save the readings for data analysis.
7. Data Analysis
- Transfer the OD600 results (growth data) into spreadsheet format. In the results section, click on the image option for each well to observe the final growth curves.
- Open the spreadsheet file and rearrange the recorded data based on the reference table. Subtract the mean value of the blank reads from the other reads.
NOTE: Blanks are the OD readings at time zero. In this protocol, OD600 values are considered for calculation. Cell density can be used if the calibration curve for OD600 vs. cell count is available.
8. Growth Rate Determination
- Use the transferred data in the spreadsheet created in step 1.6. Use the method described below and equation (1) to evaluate the growth rate.
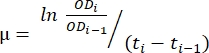 (1)
(1)
where µ is the growth rate, ODi is the OD600 in each time point, ODi-1 is the initial OD600 value in the last time point, ti and ti-1 are the time differences between the two mentioned points (e.g., 0.5 for 30 min time intervals, 1 for 1 h time intervals). - Calculate the mean and standard deviation of five successive growth rates using the desired data analysis software. Note that the largest mean with the lowest standard deviation is the maximum growth rate (μm) 12.
9. Determining Spatial Bias
NOTE: Microplate readers and plates introduce bias in results. It is crucial to assess the spatial biases of the plates used in a specific microplate reader to ensure the reliability and reproducibility of the results. To achieve that, use the following steps:
- Repeat steps 1.2-1.6.
- Record the data and analyze according to step 1.7 and step 1.8 to calculate the growth rate for each well.
- Create a heatmap to visualize the growth rate changes in the 96-well plate.
- Perform the statistical analysis (see the Supplemental Tables), ensuring no significant differences among the wells.
NOTE: It is essential to use the same type of bacteria and the same inoculum size in all wells.
10. OD reading validation and pathlength correction factor
NOTE: Determining the pathlength correction factor is crucial to validate the data and ensure reliability and validity across different devices.
- Use step 1.2 and step 1.3 to prepare the overnight inoculum of B. mycoides.
- Prepare three sterilized 50 mL flasks and add 49.5 mL of fresh TSB to each flask. Add 500 µL of the inoculum to each flask in a safety cabinet aseptically. Add 50 mL of non-inoculated TSB to a sterilized flask, which will serve as the control.
- Incubate the flasks at 30 °C and 150 rpm. Every 30 min, take samples from each flask aseptically and read and record OD600 in the spectrophotometer.
- Add 9.9 mL of TSB to a 25 mL beaker. Add 100 µL of inoculum to the beaker and shake gently.
- Add 200 µL of the mixture in the beaker to the six wells in a select column in a 96-well plate.Add 200 µL of non-inoculated TSB to three wells as a control. Fill the edge wells with 200 µL of DI water.
- Place the lid and gently transfer the 96-well plate to the microplate reader. Follow step 1.5 and step 1.6 to record the growth data.
- Plot the OD600 from the microplate reader against the OD600 from the spectrophotometer. Note that the slope is the offset between the two sets of readings, called the pathlength correction factor.
NOTE: The incubator and microplate reader incubation should be simultaneously done on the same day.
Results
OD reading validation and pathlength correction factor
Split samples of B. mycoides culture were taken at different time points and measured using the microplate reader and the spectrophotometer (Figure 1A). This step was taken to validate the results across different devices. The OD600 data correlated but did not match (Figure 1B). The correlation was linear with a slope of 0.55 (95% confidence interval [CI]: 0.53-0.58...
Discussion
Microplate readers allow for obtaining consistent and repeatable growth rates. This technology minimizes human error and enables high-throughput sampling. The small amount of culture required per sample makes this approach an attractive, low-cost alternative to cell counts using flasks or test tubes. Microplate readers allow a large sample size, increasing the statistical power and subsequently facilitating reliable growth rate calculations while keeping costs and labor low.
This article prese...
Disclosures
The authors have no conflicts of interest to declare.
Acknowledgements
This work was funded by the Natural Sciences and Engineering Research Council (NSERC) / Halifax Water Industrial Research Chair in Water Quality and Treatment (Grant No. IRCPJ 349838-16). The team of authors also would like to acknowledge the help of Anita Taylor in reviewing this article.
Materials
| Name | Company | Catalog Number | Comments |
| Centrifuge | Eppendorf | 5810 R | |
| Centrifuge tubes - 15 mL | ThermoFisher- Scientific | 339650 | Sterile |
| Centriguge tubes - 50 mL | ThermoFisher- Scientific | 339652 | Sterile |
| Disposable inoculating loop , 10 µL | Cole-Parmer | UZ-06231-08 | Sterile |
| Erlenmeyer flasks - 250 mL | Cole-Parmer | UZ-34502-59 | Glass |
| Isopropanol | ThermoFisher- Scientific | 396982500 | ≥99.0 |
| Phosphate Buffer Saline | Sigma-Aldrich | P4417 | |
| Pipett tips 1,000 µL | ThermoFisher- Scientific | UZ-25001-76 | |
| Pipett tips 10 mL | ThermoFisher- Scientific | UZ-25001-83 | |
| Pipett tips 200 µL | ThermoFisher- Scientific | UZ-25001-85 | |
| Pipett tips 5 mL | ThermoFisher- Scientific | UZ-25001-80 | |
| Pipettor 1,000 µL | Cole-Parmer | UZ-07909-11 | |
| Pipettor 10 mL | Cole-Parmer | UZ-07909-15 | |
| Pipettor 200 µL | Cole-Parmer | UZ-07909-09 | |
| Pipettor 5 mL | Cole-Parmer | UZ-07859-30 | |
| Tryptic Soy Broth | Millipore | 22091 | Suitable for microbiology |
References
- Reuß, D. R., et al. Large-scale reduction of the Bacillus subtilis genome: Consequences for the transcriptional network, resource allocation, and metabolism. Genome Research. 27 (2), 289-299 (2017).
- Sparkes, A., et al. Towards Robot Scientists for autonomous scientific discovery. Automated Experimentation. 2 (1), (2010).
- Zwietering, M. H., Jongenburger, I., Rombouts, F. M., van t Riet, K. Modeling of the bacterial growth curve. Applied and Environmental Microbiology. 56 (6), 1875-1881 (1990).
- Pla, M., Oltra, S., Esteban, M., Andreu, S., Palop, A. Comparison of primary models to predict microbial growth by the plate count and absorbance methods. BioMed Research International. 2015 (6), 1-14 (2015).
- Choe, D., et al. Adaptive laboratory evolution of a genome-reduced Escherichia coli. Nature Communications. 10 (1), 935 (2019).
- Dykhuizen, D. E., Dean, A. M. Enzyme activity and fitness: Evolution in solution. Trends in Ecology and Evolution. 5 (8), 257-262 (1990).
- McDonald, M. J. Microbial experimental evolution - A proving ground for evolutionary theory and a tool for discovery. EMBO Reports. 20 (8), 1-14 (2019).
- Lum, P. Y., et al. Discovering modes of action for therapeutic compounds using a genome-wide screen of yeast heterozygotes. Cell. 116 (1), 121-137 (2004).
- Cagnon, C., et al. Development of a forward genetic screen to isolate oil mutants in the green microalga Chlamydomonas reinhardtii. Biotechnology for Biofuels. 6 (1), 178 (2013).
- Stevenson, K., McVey, A. F., Clark, I. B. N., Swain, P. S., Pilizota, T. General calibration of microbial growth in microplate readers. Scientific Reports. 6 (1), 38828 (2016).
- Matlock, B. C., Beringer, R. W., Ash, D. L., Page, A. F., Allen, M. W. Differences in Bacterial Optical Density Measurements between Spectrophotometers. Technical Note. ThermoScientific. , (2011).
- Hall, B. G., Acar, H., Nandipati, A., Barlow, M. Growth rates made easy. Molecular Biology and Evolution. 31 (1), 232-238 (2014).
- Rolfe, M. D., et al. Lag phase is a distinct growth phase that prepares bacteria for exponential growth and involves transient metal accumulation. Journal of Bacteriology. 194 (3), 686-701 (2012).
- Djordjevic, D., Wiedmann, M., Mclandsborough, L. A. Microtiter plate assay for assessment of Listeria monocytogenes biofilm formation. Applied and Environmental Microbiology. 68 (6), 2950-2958 (2002).
- O'Toole, G. A. Microtiter dish biofilm formation assay. Journal of Visualized Experiments. (47), e2437 (2011).
- Campbell, J. High-throughput assessment of bacterial growth inhibition by optical density measurements. Current Protocols in Chemical Biology. 3 (3), 1-20 (2012).
- Toussaint, M., Conconi, A. High-throughput and sensitive assay to measure yeast cell growth: A bench protocol for testing genotoxic agents. Nature Protocols. 1 (4), 1922-1928 (2006).
- Goughenour, K. D., Balada-Llasat, J. -. M., Rappleye, C. A. Quantitative microplate-based growth assay for determination of antifungal susceptibility of Histoplasma capsulatum yeasts. Journal of Clinical Microbiology. 53 (10), 3286-3295 (2015).
- Qiu, T. A., et al. Growth-based bacterial viability assay for interference-free and high-throughput toxicity screening of nanomaterials. Analytical Chemistry. 89 (3), 2057-2064 (2017).
- Kurokawa, M., Precise Ying, B. -. W. Precise, high-throughput analysis of bacterial growth. Journal of Visualized Experiments. (127), e56197 (2017).
- Bredit, F., Romick, T. L., Fleming, H. P. A rapid method for determination of bacterial growth kinetics. Journal of Rapid Methods and Automation in Microbiology. 3, 59-68 (1994).
- Delaney, N. F., et al. Development of an optimized medium, strain and high-throughput culturing methods for Methylobacterium extorquens. PLOS ONE. 8 (4), 62957 (2013).
- Haire, T. C., et al. Robust microplate-based methods for culturing and in vivo phenotypic screening of Chlamydomonas reinhardtii. Frontiers in Plant Science. 9, 235 (2018).
- McBirney, S. E., Trinh, K., Wong-Beringer, A., Armani, A. M. Wavelength-normalized spectroscopic analysis of Staphylococcus aureus and Pseudomonas aeruginosa growth rates. Biomedical Optics Express. 7 (10), 4034 (2016).
- Sieben, M., Giese, H., Grosch, J. H., Kauffmann, K., Büchs, J. Permeability of currently available microtiter plate sealing tapes fail to fulfil the requirements for aerobic microbial cultivation. Biotechnology Journal. 11 (12), 1525-1538 (2016).
- Zimmermann, H. F., John, G. T., Trauthwein, H., Dingerdissen, U., Huthmacher, K. Rapid evaluation of oxygen and water permeation through microplate sealing tapes. Biotechnology Progress. 19 (3), 1061-1063 (2003).
- Abkar, L., Gagnon, G. A. Biological responses to P-limitation in indigenous bacteria isolated from drinking water. AWWA Water Science. 3 (5), 1248 (2021).
- Sanders, E. R. Aseptic laboratory techniques: Plating methods. Journal of Visualized Experiments. (63), e3064 (2012).
- Hart, S. F. M., Skelding, D., Waite, A. J., Burton, J. C., Shou, W. High-throughput quantification of microbial birth and death dynamics using fluorescence microscopy. Quantitative Biology. 7 (1), 69-81 (2019).
- Periago, P. M., Abee, T., Wouters, J. A. Analysis of the heat-adaptive response of psychrotrophic Bacillus weihenstephanensis. International Journal of Food Microbiology. 79 (1-2), 17-26 (2002).
- Mira, P., Barlow, M., Meza, J. C., Hall, B. G. Statistical package for growth rates made easy. Molecular Biology and Evolution. 34 (12), 3303-3309 (2017).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved