A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Microsurgical Obstruction of Testes Fusion in Spodoptera litura
In This Article
Summary
Aluminum foil was microsurgically inserted between the testes of Spodoptera litura to obstruct the fusion of testis. The procedure includes freezing, fixing, disinfection, incision, placing the barrier, suturing, postoperative feeding, and inspection. This approach provides a method to interfere with tissue formation.
Abstract
Instead of using genetic methods like RNA interference (RNAi) and clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR-associated endonuclease Cas9, a physical barrier was microsurgically inserted between the testes of Spodoptera litura to study the impact of this microsurgery on its growth and reproduction. After inserting aluminum foil between the testes, insect molting during metamorphosis proceeded normally. Insect growth and development were not remarkably altered; however, the number of sperm bundles changed if testes fusion was stopped by the microsurgery. These findings imply that blocking testicular fusion can influence male reproduction capability. The method can be further applied to interrupt communication between organs to study the function of specific signaling pathways. Compared to conventional surgery, microsurgery only requires freezing anesthetization, which is preferable to carbon dioxide anesthetization. Microsurgery also minimizes the surgery site area and facilitates wound healing. However, the selection of materials with specific functions needs further investigation. Avoiding tissue injury is crucial when making incisions during the operation.
Introduction
Fusion is a common phenomenon in tissue or organ development. Examples include dorsal closure and thorax closure in Drosophila1 and palate morphogenesis, neural tube morphogenesis, and heart morphogenesis in mice and chicken2. CRISPR and RNAi have been applied to investigate the roles of genes in the process of fusion2,3,4.
Spodoptera litura (S. litura, Lepidoptera: Noctuidae) is a detrimental polyphagous pest that is widely distributed in tropical and subtropical areas of Asia, including China4,5,6. The wide distribution of S. litura is partly attributed to its powerful reproductive capability, which is relevant to gonad development. Male infertility is one approach to control this pest. As shown in the schematic figure of testicular structure, the testes are enclosed by the testicular sheath, including the external sheath (peritoneal sheath) and inner basal lamina. The basal lamina extends internally to form the follicular epithelium and separates the inner area of the testis into four chambers named follicles (Figure 1).
In the follicles, spermatogonia develop into spermatozoa after mitosis and meiosis, and then the spermatozoa in the sperm sacs align in the same direction to form sperm bundles7. During spermatogenesis, the primary spermatocytes differentiate into eupyrene sperms or apyrene sperms. Spermatocytes in the larval phase develop into eupyrene sperm with a long tail connected to a head of an elongated nucleus; these can fertilize eggs. Conversely, spermatocytes in the mid-pupal phase develop into apyrene sperm with a discarded nucleus; these sperm assist the survival, motion, and fertilization of eupyrene sperm9,10. The 6th day of the pupa is the period during which the testes have abundant eupyrene and apyrene sperm bundles.
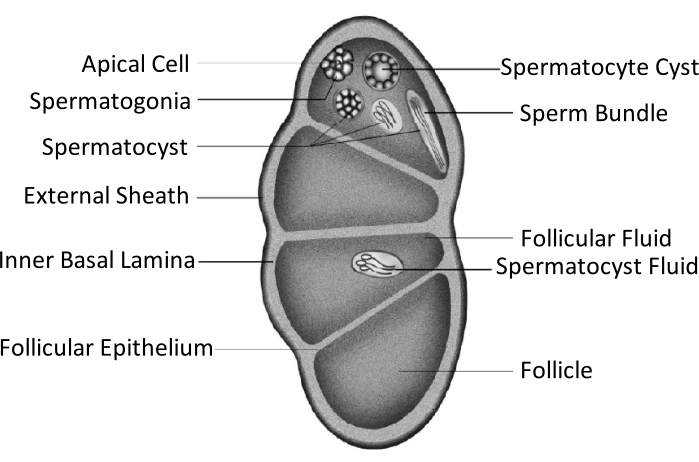
Figure 1: Schematic diagram of the testicular structure of Lepidoptera insects11. Please click here to view a larger version of this figure.
Testicular fusion occurs in most insects of the Lepidoptera order11,12, especially in those species that are agricultural pests. Testicular fusion refers to a pair of testes growing bilaterally in the larval phase, approaching and adhering to each other, eventually integrating into a single gonad11. In Spodoptera litura, it happens during metamorphosis from the larval to the pupal stage. From day 1 of the 5th instar (L5D1) to day 4 of the 6th instar (L6D4), the pair of testes grows gradually in size, and the color turns light yellow from ivory-white. It becomes faint red as it reaches the prepupal phase (L6D5 to L6D6). Two bilateral symmetrical testes approach each other during the prepupal stage, fuse into one, and twist anticlockwise (doral view) to produce a single testis in the pupal and adult phases11. This phenomenon does not occur in silkworms, which have considerable economic importance and have been domesticated for 5000 years13. Thus, it is assumed that the testes' fusion improves reproductive capability.
To determine the significance of Spodoptera litura testicular fusion, it is important to investigate the effects of blocking the process. In this protocol, aluminum foil was microsurgically inserted between the testes to keep them separated, and the consequent changes in the development of the insects and their testes were studied.
Protocol
1. Insect rearing and maintenance
- Culture the Spodoptera litura larvae in environmental simulation chambers with an artificial diet until they reach day 4 of the 6th instar (L6D4). Select male larvae when the worms enter the first day of the 6th instar (L6D0) based on the inverse triangle-shaped structure on the eighth abdomen14.
NOTE: Larvae rearing and maintenance techniques were published previously4,14.
2. Presurgical preparation
- Trim the aluminum foil into rectangular pieces with rounded corners (1 mm x 2 mm, Figure 2).
- Sterilize the surgery platform and related items (table surface, microscope, icebox, insect box, wax tray, pins, and thread) by spraying 75% alcohol on their surface and wiping them down.
- Sterilize surgical tools (including the aluminum foil) with a high-pressure steam sterilizer for 30 min, and place them in a heating and drying oven at 120 °C.
- Ensure that the operators wear clean laboratory clothes, surgical masks, and sterile gloves.
3. Microsurgical placement of a barrier between the testes
NOTE: The general work-flow is as follows: Freezing → Fixing → Disinfection → Incision → Barrier Placement → Suturing→ Postoperative Feeding and Inspection
- Place male larvae (L6D4) on ice for 10-30 min to keep them anesthetized during the operation.
- Place a larva on the wax tray with the dorsal side up, and then fix the head and the tail of the larva with pins and threads, showing the surgical area that is the dorsal surface on the 9th body segment (Figure 3A).
- Disinfect the surgical area by applying 3% iodine tincture with a cotton swab to the epidermis (9th body segment), followed by 70% alcohol to remove the iodine (Figure 3B).
NOTE: Focus on the larva through coarse and fine adjustment of the surgical microscope (Figure 3C). Place the wax tray on a larger culture dish filled with ice to keep the anesthesia. - Make a 2 mm-long incision on the dorsal epidermis of the 9th body segment. Next, use a sterile cotton swab to remove any leaking hemolymph and fat bodies and obtain a clear view of the surgical area.
NOTE: It is important to avoid the heart during the procedure. This can be done by making the incision slightly next to the mid-line in the 9th body segment or at the joint between the 9th and 10th body segments to prevent the testes from popping out due to the larval internal pressure. While using the scalpel, make a vertical slit with the blade first (Figure 4A), and then turn it 45° towards the epidermis before evenly and continuously cutting through the epidermis (Figure 4B). - Use surgical tweezers to insert a piece of aluminum foil between the testes (Figure 5).
- At the end of the surgery, close the incision to avoid infection, and allow the larvae to recover from the surgery.
- Close the epidermis with a running suture (Figure 6).
- Use a needle holder and surgical tweezers to tie a surgical square knot, requiring two opposing mirror-image simple knots (Figure 6D,E).
- Use scissors to cut the excess suture from the loop tails, leaving a 2 mm thread behind.
- After suturing, gently lay the larva in the rearing box and maintain them in a clean environmental simulation chamber. Continue observing the larvae.
NOTE: The wound stops leaking hemolymph, and the larvae gradually recover after the surgery. The worms continue to complete their metamorphosis.
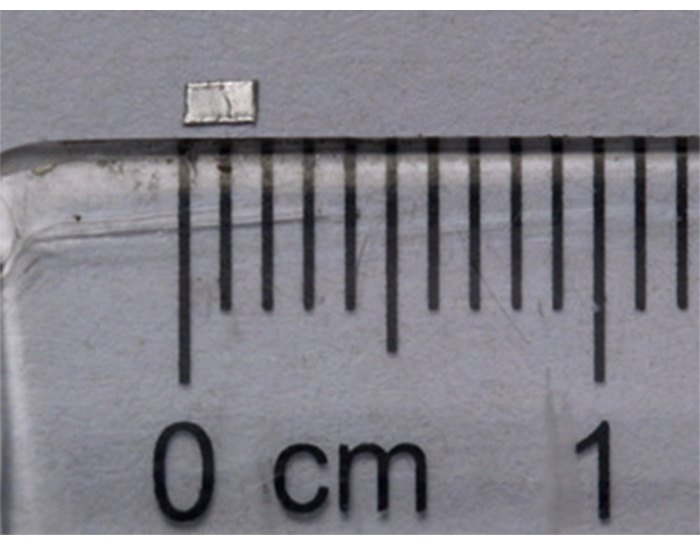
Figure 2: Physical barrier prepared using aluminum foil (1 mm x 2 mm). Please click here to view a larger version of this figure.
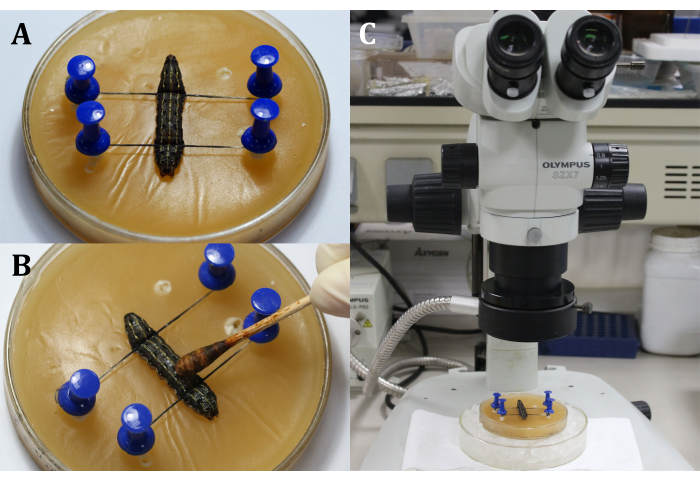
Figure 3: Before incision. (A) Fixing the larva. (B) Disinfection of the epidermis of the surgical area. (C)Performing surgery under the microscope. Please click here to view a larger version of this figure.
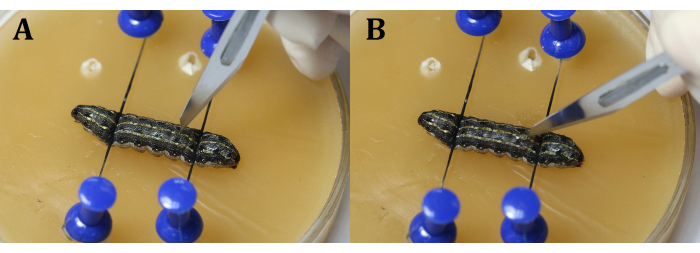
Figure 4: Incision. (A) Slit the larvae vertically with the blade. (B) Turn the blade 45° toward the epidermis before cutting through. Please click here to view a larger version of this figure.
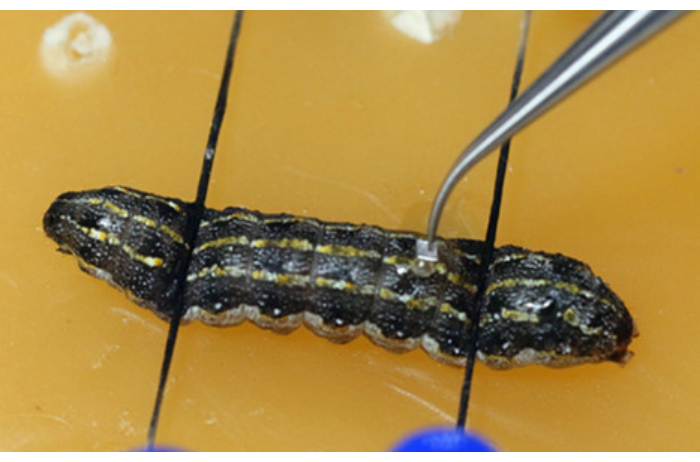
Figure 5: Inserting the physical barrier (aluminum foil) between the testes. Please click here to view a larger version of this figure.
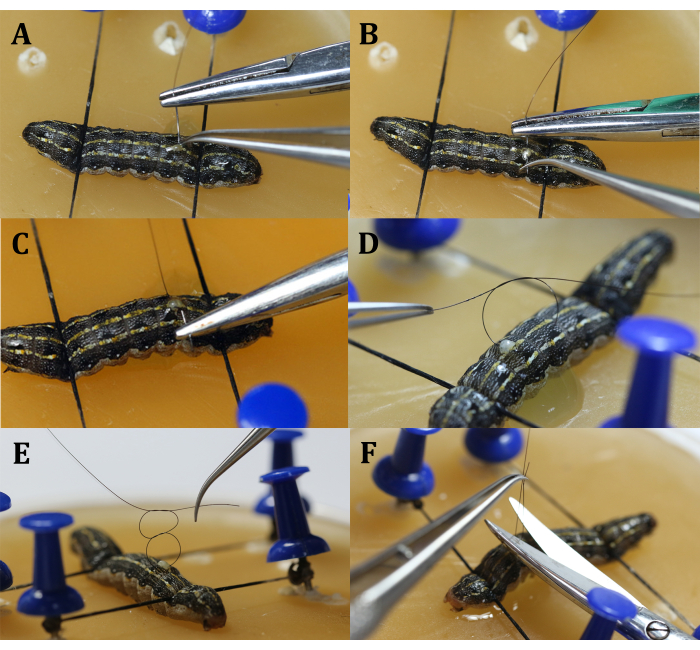
Figure 6: Suturing. (A) Insert the needle. (B) Withdraw the needle. (C) Withdraw and clamp the needle. (D) Tie the first simple knot. (E) Tie the opposing mirror-imaged simple knot. (F) Cut excess suture thread. Please click here to view a larger version of this figure.
Results
The effects of microsurgery on Spodoptera litura growth and development
The microsurgery left a 2 mm-long wound on the dorsal larval epidermis that eventually stopped leaking hemolymph and healed. The larvae went through prepupal and pupal stages and eclosed, indicating that the microsurgery had no major impact on growth and development. When the larvae molted into pupae, the suture threads were discarded along with the epidermis. There were no obvious dif...
Discussion
After microsurgically obstructing testes fusion in Spodoptera litura, the number of sperm bundles decreased, which supported the hypothesis that this fusion is beneficial to the reproductive capability. Surgical manipulation has been used to study the physiological development of insects since the early 20th century. To determine whether the cranial nerve is regulated by insect metamorphosis, some researchers performed procedures such as ligation and decapitation on different insects (including Rh...
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work were supported by National Natural Science Foundation of China (Nos.:31772519, 31720103916; ) and an open grant from the State Key Laboratory of Silkworm Genome Biology, South West University (No.: sklsgb2013003).
Materials
| Name | Company | Catalog Number | Comments |
| 75% Rubbing alcohol | Qingdao Hainuo Nuowei Disinfection Technology Co., Ltd | Q/370285HNW 001-2019 | |
| Colored Push Pins | Deli Group Co.,LTD | 0042 | |
| Corneal Scissors | Suzhou Xiehe Medical Device Co., Ltd | MR-S221A | Curved and blunt tip |
| Glad Aluminum Foil | Clorox China(Guangzhou) Limited | 831457 | 10 cm*2.5 cm*0.6 |
| Medical Cotton Swabs (Sterile) | Winner Medical Co., Ltd. | 601-022921-01 | |
| Medical Iodine Cotton Swab | Winner Medical Co., Ltd. | 608-000247 | |
| Needle holder | Shanghai Medical Instruments (Group) Ltd., Corp. | J32030 | 14 cm fine needle |
| Sterile surgical blade | Shanghai Pudong Jinhuan Medical Supplies Co., LTD | #11 | |
| Suigical Blade Holder | Shanghai Pudong Jinhuan Medical Supplies Co., LTD | K6-10 | Straight 3# |
| Suture thread with needle | Ningbo Medical Stitch Needle Co., Ltd | needle: 3/8 Circle, 2.5*8 ; Thread: Nylon, 6/0, 25 cm | |
| Tying Forceps | Suzhou Xiehe Medical Device Co., Ltd | MR-F201T-3 | Straight-pointed; long handle; 0.12 mm-wide-head |
References
- Zeitlinger, J., Bohmann, D. Thorax closure in Drosophila: involvement of Fos and the JNK pathway. Development. 126 (17), 3947-3956 (1999).
- Ray, H. J., Niswander, L. Mechanisms of tissue fusion during development. Development. 139 (10), 1701-1711 (2012).
- Ducuing, A., Keeley, C., Mollereau, B., Vincent, S. A. DPP-mediated feed-forward loop canalizes morphogenesis during Drosophila dorsal closure. The Journal of Cell Biology. 208 (2), 239-248 (2015).
- Du, Q., et al. Identification and functional characterization of doublesex gene in the testis of Spodoptera litura. Insect Science. 26 (6), 1000-1010 (2019).
- Qin, H. G., Ding, J., Ye, Z. X., Huang, S. J., Luo, R. H. Dynamic analysis of experimental population of Spodoptera litura. Journal of Biosafety. 13 (2), 45-48 (2004).
- Guan, B. B., et al. The research in biology and ecology of Spodoptera litura. Journal of Biosafety. 8 (1), 57-61 (1999).
- Wen, L., et al. The testis development and spermatogenesis in Spodopture litura (lepidoptera: noctuidae). Journal of South China Normal University (Natural Science Edition. 51 (4), 47-56 (2019).
- Friedländer, M., Seth, R. K., Reynolds, S. E. Eupyrene and apyrene sperm: dichotomous spermatogenesis in Lepidoptera. Advances in Insect Physiology. 32, 206 (2010).
- Cook, P. A., Wedell, N. Non-fertile sperm delay female remating. Nature. 397 (6719), 486 (1999).
- Iriki, S. On the function of apyrene spermatozoa in the silk worm. Zoological Magazine. 53, 123-124 (1941).
- Liu, L., Feng, Q. L. The study of fusion of testis in Lepidoptera insects. Journal of South China Normal University (Natural Science Edition). 46 (5), 1-7 (2014).
- Klowden, M. J. . Physiological systems in insects. , (2007).
- Xu, J., et al. Transgenic characterization of two testis-specific promoters in the silkworm, Bombyx mori. Insect Molecular Biology. 24 (2), 183-190 (2015).
- Guo, X. R., Zheng, S. C., Liu, L., Feng, Q. L. The sterol carrier protein 2/3-oxoacyl-CoA thiolase (SCPx) is involved in cholesterol uptake in the midgut of Spodoptera litura: gene cloning, expression, localization and functional analyses. BioMed Central Molecular Biology. 10, 102 (2009).
- Kopeć, S. Studies on the necessity of the brain for the inception of insect metamorphosis. Biological Bulletin. 42 (6), 323-342 (1922).
- Wigglesworth, V. B. Factors controlling moulting and 'metamorphosis' in an insect. Nature. 133 (5), 725-726 (1934).
- Williams, C. N. Physiology of insect diapause; the role of the brain in the production and termination of pupal dormancy in the giant silkworm, Platysamia cecropia. Biological Bulletin. 90 (3), 234-243 (1946).
- Fukuda, S. Hormonal control of molting and pupation in the silkworm. Proceedings of the Imperial Academy Tokyo. 16 (8), 417-420 (1940).
- Tian, H. J., Liu, Z. P., Bai, Y. Y. Common methods to detect Sperm quality of mammalian. Journal of Economic Zoology. 8 (4), 198-201 (2004).
- Ji, X. S., Chen, S. L., Zhao, Y., Tian, Y. S. Progress in the quality evaluation of fish sperm. Chinese Fishery Science. 14 (6), 1048-1054 (2007).
- Baulny, B. O. D., Vern, Y. L., Kerboeuf, D., Maisse, G. Flow cytometric evaluation of mitochondrial activity and membrane integrity in fresh and cryopreserved rainbow trout (Oncorhynchus mykiss) spermatozoa. Cryobiology. 34 (2), 141-149 (1997).
- Krasznai, Z., Márián, T., Balkay, I., Emri, M., Trón, L. Permeabilization and structural changes in the membrane of common carp (Cyprinus carpio L.) sperm induced by hypo-osmotic shock. Aquaculture. 129 (1), 134 (1995).
- Kime, D. E., et al. Computer-assisted sperm analysis (CASA) as a tool for monitoring sperm quality in fish. Comparative Biochemistry & Physiology Toxicology & Pharmacology Cbp. 130 (4), 425-433 (2001).
- Rurangwa, E., Volckaert, F. A., Huyskens, G., Kime, D. E., Ollevier, F. Quality control of refrigerated and cryopreserved semen using computer-assisted sperm analysis (CASA), viable staining and standardized fertilization in African catfish (Clarias gariepinus). Theriogenology. 55 (3), 751-769 (2001).
- Seth, R. K., Kaur, J. J., Rao, D. K., Reynolds, S. E. Effects of larval exposure to sublethal concentrations of the ecdysteroid agonists RH-5849 and tebufenozide (RH-5992) on male reproductive physiology in Spodoptera litura. Journal of Insect Physiology. 50 (6), 505-517 (2004).
- Sweeney, R. M., Watterson, R. L. Rib development in chick embryos analyzed by means of tantalum foil blocks. American Journal of Anatomy. 126 (2), 127-149 (1969).
- Wilde, S., Logan, M. P. Application of impermeable barriers combined with candidate factor soaked beads to study inductive signals in the chick. Journal of Visualized Experiments. (117), e54618 (2016).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved